Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**Dosage and Administration** Pharmaceutical Form Concentrate for solution for infusion. **Posology** The recommended dose as monotherapy is 500 mg _JEMPERLI_ administered as an intravenous infusion over 30 minutes every 3 weeks for 4 doses followed by 1000 mg every 6 weeks for all cycles thereafter. The dosage regimen is presented in Table 1. 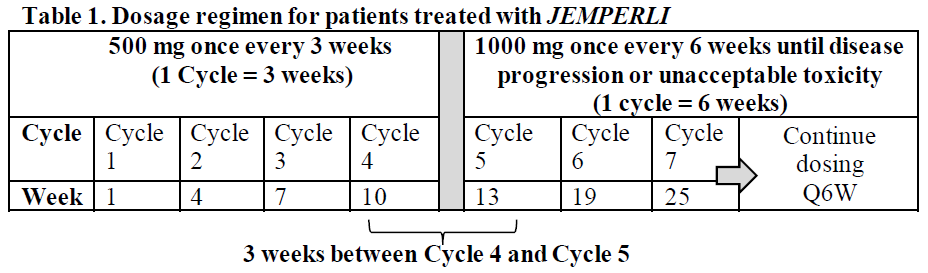 Administration of _JEMPERLI_ should continue according to the recommended dose and schedule until disease progression or unacceptable toxicity. _Dose modifications_ Dose reduction is not recommended. Dosing delay or discontinuation may be required based on individual safety and tolerability. Recommended modifications to manage adverse reactions are provided in Table 2. Detailed guidelines for the management of immune-related adverse reactions and infusion-related reactions are described in _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. 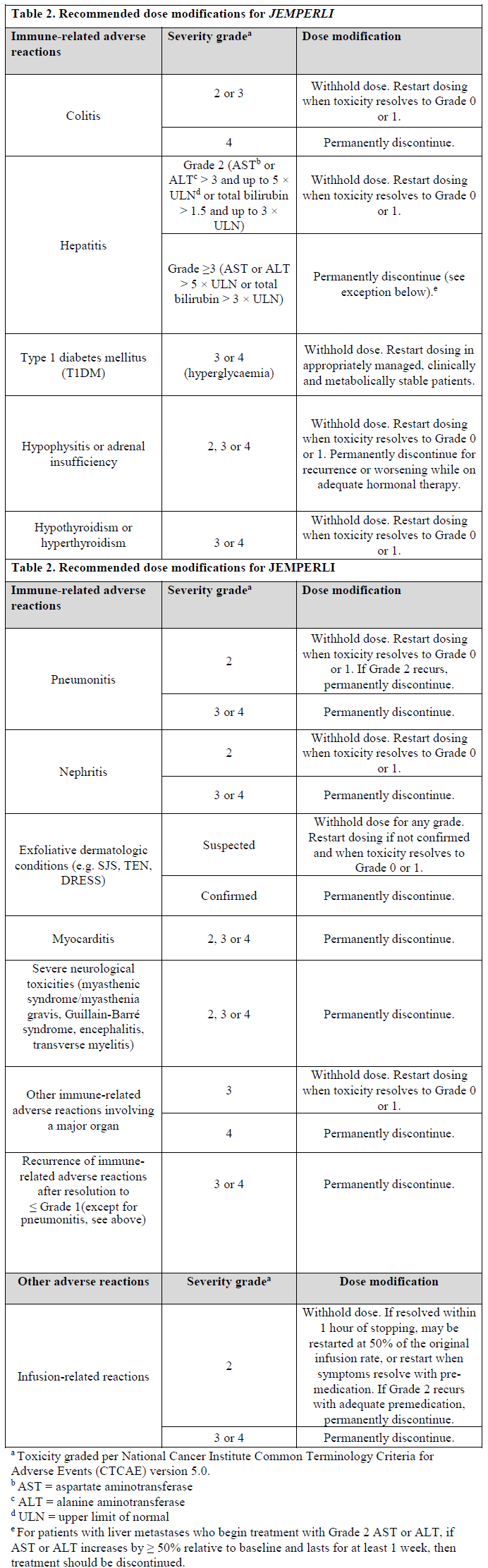 **Method of Administration** _JEMPERLI_ is for intravenous infusion only. _JEMPERLI_ should be administered by intravenous infusion using an intravenous infusion pump over 30 minutes. _JEMPERLI_ must not be administered as an intravenous push or bolus injection. For instructions on dilution of the medicinal product before administration, _see Use and Handling_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Children** The safety and efficacy of _JEMPERLI_ in children and adolescents aged under 18 years have not been established. No data are available. **Elderly** No dose adjustment is recommended for patients who are aged 65 years of age or over. There are limited clinical data with _JEMPERLI_ in patients aged 75 years or over. **Renal impairment** No dose adjustment is recommended for patients with mild or moderate renal impairment. There are limited data in patients with severe renal impairment or end-stage renal disease undergoing dialysis ( _see Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No dose adjustment is recommended for patients with mild hepatic impairment. There are limited data in patients with moderate hepatic impairment and no data in patients with severe hepatic impairment ( _see Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**Indications** _JEMPERLI_ is indicated as monotherapy for the treatment of adult patients with recurrent or advanced mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) endometrial cancer (EC) that has progressed on or following prior treatment with a platinum-containing regimen.
**Contraindications** Hypersensitivity to the active substance or to any of the excipients ( _see List of Excipients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01FF07
dostarlimab
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
Ajinomoto Althea Incorporated
Active Ingredients
Documents
Package Inserts
Jemperli Concentrate for Solution for Infusion PI.pdf
Approved: October 6, 2022
