Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**4.2 Posology and method of administration** Posology MRI of the liver: the recommended dose of MultiHance injection in adult patients is 0.05 mmol/kg body weight. This corresponds to 0.1 mL/kg of the 0.5 M solution. MRI of the brain and spine: the recommended dose of MultiHance injection in adult and in paediatric patients greater than 2 years of age is 0.1 mmol/kg body weight. This corresponds to 0.2 mL/kg of the 0.5 M solution. MRA: the recommended dose of MultiHance injection in adult patients is 0.1 mmol/kg body weight. This corresponds to 0.2 mL/kg of the 0.5 M solution. MRI of the breast: the recommended dose of MultiHance in adult patients is 0.1 mmol/kg body weight. This corresponds to 0.2 mL/kg of the 0.5 M solution. Method of administration MultiHance should be drawn up into the syringe immediately before use and should not be diluted. Any unused product should be discarded and not be used for other MRI examinations. To minimise the potential risks of soft tissue extravasation of MultiHance, it is important to ensure that the i.v. needle or cannula is correctly inserted into a vein. Liver and Brain and Spine: the product should be administered intravenously either as a bolus or slow injection (10 mL/min.). MRA: the product should be administered intravenously as a bolus injection, either manually or using an automatic injector system. The injection should be followed by a saline flush. Post-contrast imaging acquisition: 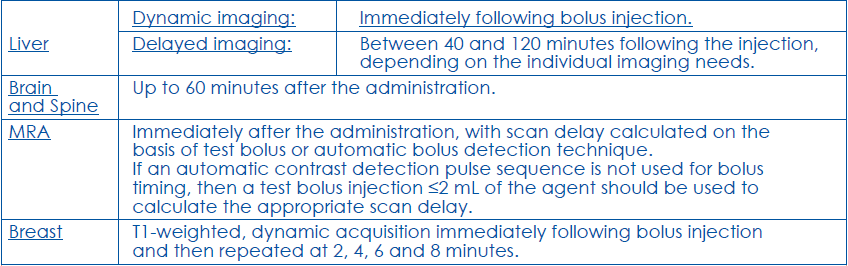 Special Populations Impaired renal function Use of MultiHance should be avoided in patients with severe renal impairment (GFR < 30 ml/min/1.73m2) and in patients in the perioperative liver transplantation period unless the diagnostic information is essential and not available with non-contrast enhanced MRI (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If use of MultiHance cannot be avoided, the dose should not exceed 0.1 mmol/kg body weight when used for MR of the brain and spine, MR-angiography or breast MRI and should not exceed 0.05 mmol/kg body weight when used for MR of the liver. More than one dose should not be used during a scan. Because of the lack of information on repeated administration, MultiHance injections should not be repeated unless the interval between injections is at least 7 days. Elderly (aged 65 years and above) No dosage adjustment is considered necessary. Caution should be exercised in elderly patients (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Pediatric population No dosage adjustment is considered necessary. Use for MRI of the brain and spine is not recommended in children less than 2 years of age. Use for MRI of the liver, MRI of the breast or MRA is not recommended in children less than 18 years of age.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** This medicinal product is for diagnostic use only. MultiHance is a paramagnetic contrast agent for use in diagnostic magnetic resonance imaging (MRI) indicated for: - MRI of the liver for the detection of focal liver lesions in patients with known or suspected primary liver cancer (eg. hepatocellular carcinoma) or metastatic disease. - MRI of the brain and spine where it improves the detection of lesions and provides diagnostic information additional to that obtained with unenhanced MRI. - Contrast-enhanced MR- angiography where it improves the diagnostic accuracy for detecting clinically significant steno-occlusive vascular disease in patients with suspected or known vascular disease of the abdominal or peripheral arteries. - MRI of the breast, for the detection of malignant lesions in patients where breast cancer is known or suspected on the basis of previous mammography or ultrasound results.
**4.3 Contra-indications** MultiHance is contra-indicated in: - patients with hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - patients with a history of allergic or adverse reactions to other gadolinium chelates.
V08CA08
gadobenic acid
Manufacturer Information
DCH AURIGA SINGAPORE
Patheon Italia S.P.A.
Active Ingredients
Documents
Package Inserts
Multihance Injection PI.pdf
Approved: January 24, 2022
