Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**How should Otrivin 0.05% be used?** Unless otherwise prescribed by the doctor, always give Otrivin 0.05% exactly as recommended below. Do not exceed the recommended dosage, especially in children and the elderly. You should check with your doctor or pharmacist if you are not sure. Do not exceed 3 applications daily into each nostril. It is recommended to make the last application shortly before retiring to bed. The usual doses are as follows:  1. Gently clear your child’s nose. 2. Before using, practice using the dropper to develop good dosage control. 3. Tilt your child’s head slightly back, as far as is comfortable, or if lying on a bed, hang the head over the side. 4. Without touching the dropper to the nose, very carefully apply the drop(s) into each nostril and keep the head tilted back for a short time to allow the drops to spread throughout the nose. 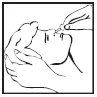 5. If the drop completely misses the child’s nose, administer the drop again. 6. If any part of the drop gets into the nose, do not administer the drop again. 7. Repeat with the other nostril. 8. Clean and dry the dropper before replacing it back into the bottle right after use. 9. To avoid possible spread of infection, the bottle should only be used by one person. Please keep to the doses which appear in the package leaflet or prescribed by your doctor. If you feel that the medicinal product is not effective enough or on the contrary, it is too effective, please consult your doctor or pharmacist. Like other nasal decongestants, Otrivin 0.05% should not be used for more than 7 consecutive days. If symptoms persist, consult your doctor. Prolonged or excessive use may cause stuffiness in the nose to return or worsen.
NASAL
Medical Information
**What is Otrivin 0.05% and when is it used?** Otrivin 0.05% is a clear, colourless and practically odourless solution containing 0.5mg/mL of xylometazoline hydrochloride. It quickly decongests your child’s stuffy nose and helps clear blocked mucus secretions so your child can breathe easier. Otrivin 0.05% is used for various types of colds. It is designed to be applied in the nostrils, where it exerts a vasoconstricting effect thereby decongesting the mucosae of the nose and the throat. This effect allows patients with colds to breathe more easily through the nose. The action of Otrivin 0.05% is felt within 2 minutes and lasts for several hours. Otrivin 0.05% contains ingredients which help prevent the nasal mucosa from drying out. Otrivin 0.05% is indicated in children aged 2 to 11 years old.
**When must Otrivin 0.05% not be used?** **Do not use Otrivin 0.05% if your child:** - is allergic to xylometazoline or any other ingredients of this medicine - has undergone recent trans-nasal surgery (brain surgery where the operation was carried out through the nose or mouth) - has narrow angle glaucoma (increased pressure in eyes) - has chronic nasal inflammation with very dry nasal passages (rhinitis sicca or atrophic rhinitis) - is below 2 years of age Tell your doctor or pharmacist if any of the above apply because Otrivin 0.05% is not suitable for use in these circumstances.
R01AA07
xylometazoline
Manufacturer Information
gsk consumer healthcare singapore pte. ltd.
GSK Consumer Health SARL
Active Ingredients
Documents
Patient Information Leaflets
OTRIV DRPS 0.05% F5 10 ML SEA PIL.pdf
Approved: September 8, 2021
