Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Dose and method of administration** **Dosage** Calculation of the cumulative iron need _Iron replacement in patients with iron deficiency:_ The dose of Monofer is expressed in mg of elemental iron. The iron need and the administration schedule for Monofer must be individually established for each patient. The optimal Hb target level and iron stores may vary in different patient groups and between patients. Please refer to official guidelines. Iron deficiency anaemia will not appear until essentially all iron stores have been depleted. Iron therapy should therefore replenish both Hb iron and iron stores. After the current iron deficit has been corrected, patients may require continued therapy with Monofer to maintain target levels of Hb and acceptable limits of other iron parameters. The cumulative iron need can be determined using either the Ganzoni formula (1) or the Table below (2). It is recommended to use the Ganzoni formula in patients who are likely to require individually adjusted dosing such as patients with anorexia nervosa, cachexia, obesity, pregnancy or anaemia due to bleeding. 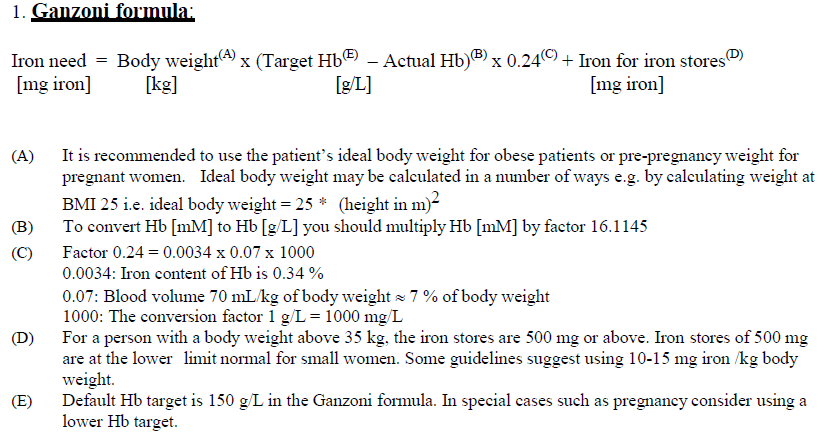 The dose-response relationship observed with Monofer suggests that the true iron demand of IV iron is underestimated by the Ganzoni formula if a Hb target of less than 150 g/L is used. 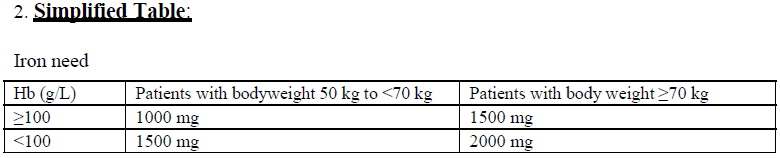 **Method of administration** Monofer must only be mixed with sterile 0.9 % sodium chloride. No other intravenous dilution solutions should be used. No other therapeutic agents should be added. The diluted solution for injection should be visually inspected prior to use. Use only clear solutions without sediment. Monitor carefully patients for signs and symptoms of hypersensitivity reactions during and following each administration of Monofer. Each IV iron administration is associated with a risk of a hypersensitivity reaction. Thus, to minimise risk the number of single IV iron administrations should be kept to a minimum. Monofer offers the flexibility of administration either as an intravenous bolus injection, as an intravenous drip infusion or as a direct injection into the venous limb of the dialyser. Monofer should not be administered concomitantly with oral iron preparations, since the absorption of oral iron might be decreased. _Intravenous bolus injection:_ Monofer may be administered as an intravenous bolus injection up to 500 mg up to three times a week at an administration rate of up to 250 mg iron/minute. It may be administered undiluted or diluted in maximum 20 mL sterile 0.9 % sodium chloride. _Intravenous drip infusion:_ The cumulative iron dose required may be administered in a single Monofer infusion up to 20 mg iron/kg body weight or as weekly infusions until the cumulative iron dose has been administered. If the cumulative iron dose exceeds 20 mg iron/kg body weight, the dose must be split in two administrations with an interval of at least one week. It is recommended whenever possible to give 20 mg iron/kg body weight in the first administration. Dependent on clinical judgement the second administration could await follow-up laboratory tests. Doses up to 1000 mg must be administered over 20 minutes. Doses exceeding 1000 mg must be administered over 30 minutes or more. Single doses above 1500 mg are not recommended. Monofer should be added to maximum 500 mL sterile 0.9 % sodium chloride. _Injection into dialyser:_ Monofer may be administered during a haemodialysis session directly into the venous limb of the dialyser under the same procedures as outlined for intravenous bolus injection.
INTRAVENOUS DRIP, INTRAVENOUS BOLUS
Medical Information
**4.1 Therapeutic indications** Monofer is indicated for the treatment of iron deficiency in adults, under the following conditions: - When oral iron preparations are ineffective or cannot be used - Where there is a clinical need to deliver iron rapidly The diagnosis must be based on laboratory tests.
**4.3 Contraindications** - Hypersensitivity to the active substance, to Monofer or any of the excipients - Known serious hypersensitivity to other parental iron products - Non-iron deficiency anaemia (eg. haemolytic anaemia) - Iron overload or disturbances in utilisation of iron (Eg. haemochromatosis, haemosiderosis) - Decompensated liver cirrhosis or active hepatitis
B03AC
非肠道用药的三价铁制剂
Manufacturer Information
COMPAI PHARMA PTE. LTD.
Wasserburger Arzneimittelwerk GmbH
Active Ingredients
Documents
Package Inserts
Monofer Solution for Injection or Infusion PI.pdf
Approved: November 5, 2021
