Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**DOSAGE REGIMEN AND ADMINISTRATION** **Monitoring instructions** **Blood cell counts:** a blood cell count must be performed before initiating therapy with Jakavi. Complete blood counts should be monitored every 2 to 4 weeks until doses are stabilized, and then as clinically indicated (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Starting dose** The recommended starting dose of Jakavi in myelofibrosis (MF) is based on platelet counts (see Table 1): 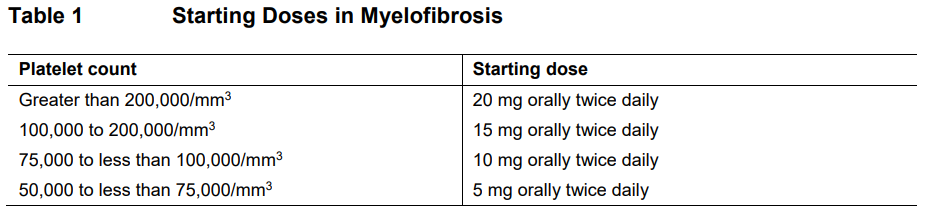 The recommended starting dose of Jakavi in Polycythemia vera (PV) is 10 mg given orally twice daily. The recommended starting dose of Jakavi in acute Graft-versus-host disease (aGvHD) is 5 to 10 mg given orally twice daily. The recommended starting dose of Jakavi in chronic Graft-versus-host disease (cGvHD) is 10 mg given orally twice daily. In aGvHD and cGvHD, Jakavi can be added to the continued use of corticosteroids and/or calcineurin inhibitors (CNIs). **Dose modifications** Doses may be titrated based on efficacy and safety. If efficacy is considered insufficient and blood counts are adequate, doses may be increased by a maximum of 5 mg twice daily, up to the maximum dose of 25 mg twice daily. The starting dose should not be increased within the first four weeks of treatment and thereafter no more frequently than at 2-week intervals. _Myelofibrosis and Polycythemia vera_ Treatment should be interrupted for platelet counts less than 50,000/mm3 or absolute neutrophil counts less than 500/mm3. In PV, treatment should also be interrupted when hemoglobin is below 8 g/dL. After recovery of blood counts above these levels, dosing may be restarted at 5 mg twice daily and gradually increased based on careful monitoring of blood cell counts. Dose reductions should be considered if the platelet counts decrease during treatment as outlined in Table 2, with the goal of avoiding dose interruptions for thrombocytopenia. 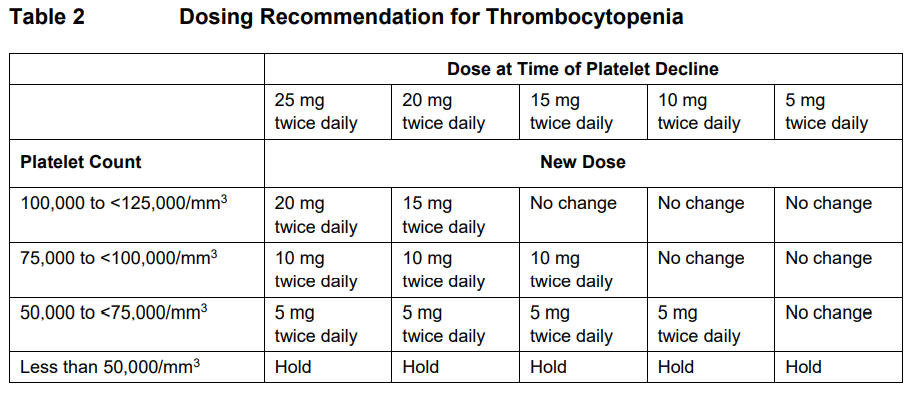 In PV, dose reduction should also be considered if hemoglobin decreases below 12 g/dL and is recommended if hemoglobin decreases below 10 g/dL. _Acute Graft-versus-host disease_ The recommended starting dose of Jakavi in acute GvHD is 5 to 10 mg given orally twice daily with or without food. _Chronic Graft-versus-host disease_ The recommended starting dose of Jakavi in chronic GvHD is 10 mg given orally twice daily with or without food. _Dose modifications for acute and chronic GvHD_ Dose reductions and temporary interruptions of treatment may be needed in GvHD patients with thrombocytopenia, neutropenia, or elevated total bilirubin after standard supportive therapy including growth-factors, anti-infective therapies and transfusions. One dose level reduction step is recommended (10 mg twice daily to 5 mg twice daily or 5 mg twice daily to 5 mg once daily). In patients who are unable to tolerate Jakavi at a dose of 5 mg once daily, treatment should be interrupted. Detailed dosing recommendations are provided in Table 3. 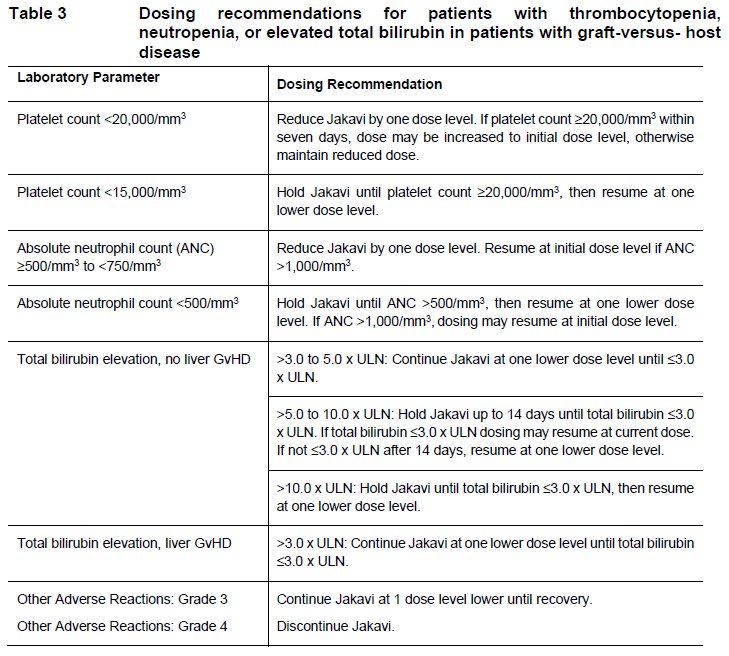 _Administration instruction_ If a dose is missed, the patient should not take an additional dose, but should take the next usual prescribed dose. Treatment of MF and PV may be continued as long as the benefit: risk ratio remains positive. In GvHD, tapering of Jakavi may be considered in patients with a response and after having discontinued corticosteroids. A 50% dose reduction of Jakavi every two months is recommended. If signs or symptoms of GvHD reoccur during or after the taper of Jakavi, re-escalation of treatment should be considered. **Dose adjustment with concomitant strong CYP3A4 Inhibitors or fluconazole** When Jakavi is administered with strong CYP3A4 inhibitors in MF and PV patients or dual moderate inhibitors of CYP2C9 and CYP3A4 enzymes (e.g. fluconazole) in MF, PV or GvHD patients, the unit dose of Jakavi should be reduced by approximately 50%, to be administered twice daily. The concomitant use of Jakavi with fluconazole doses greater than 200 mg daily should be avoided (see section INTERACTION – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). More frequent monitoring of hematology parameters and clinical signs and symptoms of Jakavi related adverse drug reactions (ADRs) is recommended while on a strong CYP3A4 inhibitor or dual moderate inhibitors of CYP2C9 and CYP3A4 enzymes. **Special populations** **Renal impairment** In patients with severe renal impairment (creatinine clearance (Clcr) less than 30 mL/min) the recommended starting dose based on platelet count for MF patients should be reduced by approximately 50%. The recommended starting dose for PV patients with severe renal impairment is 5 mg twice daily. Patients diagnosed with severe renal impairment while receiving Jakavi should be carefully monitored and may need to have their doses reduced to avoid ADRs. There are limited data to determine the best dosing options for patients with end-stage renal disease (ESRD) on dialysis. Available data in this population suggest that MF patients on dialysis should be started on an initial single dose of 15 mg or 20 mg based on platelet counts with subsequent single doses only after each dialysis session, and with careful monitoring of safety and efficacy. The recommended starting dose for PV patients with ESRD on hemodialysis is a single dose of 10 mg or two doses of 5 mg given 12 hours apart, to be administered post-dialysis and only on the day of haemodialysis. These dose recommendations are based on simulations and any dose modification in ESRD should be followed by careful monitoring of safety and efficacy in individual patients. No data is available for dosing patients who are undergoing peritoneal dialysis or continuous venovenous haemofiltration (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 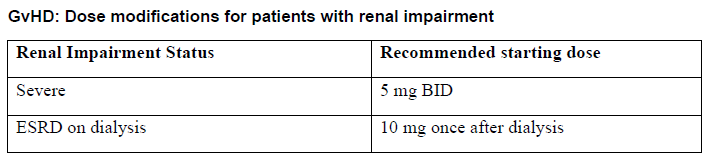 **Hepatic Impairment** In MF and PV patients with any hepatic impairment the recommended starting dose based on platelet counts should be reduced by approximately 50% to be administered twice daily. Subsequent doses should be adjusted based on careful monitoring of safety and efficacy. Patients diagnosed with hepatic impairment while receiving Jakavi should have complete blood counts, including a white blood cell count differential, monitored at least every one to two weeks for the first 6 weeks after initiation of therapy with Jakavi and as clinically indicated thereafter once their liver function and blood counts have been stabilised. Jakavi dose can be titrated to reduce the risk of cytopenia. 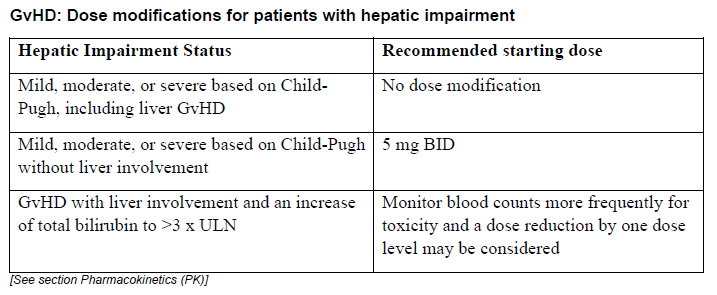 **Pediatrics** The safety and efficacy of Jakavi in pediatric patients with MF and PV have not been established. In pediatric patients (12 years of age and older) with GvHD, the safety and efficacy of Jakavi are supported by evidence from the randomized phase 3 studies REACH2 and REACH3. The Jakavi dose in pediatric patients with GvHD aged 12 years and older is the same as in adults. The safety and efficacy of Jakavi have not been established in patients less than 12 years of age. **Geriatrics** No additional dose adjustments are recommended for elderly patients. **Method of administration** Jakavi is dosed orally and can be administered with or without food.
ORAL
Medical Information
**INDICATIONS** _Myelofibrosis_ Jakavi is indicated for the treatment of disease-related splenomegaly and/or symptoms in adult patients with myelofibrosis, including primary myelofibrosis, post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis. _Polycythemia vera_ Jakavi is indicated for the treatment of adult patients with polycythemia vera who are resistant to or intolerant of hydroxyurea. _Acute Graft-versus-host disease_ Jakavi is indicated for the treatment of patients with acute Graft versus Host Disease (aGvHD) aged 12 years and older who have inadequate response to corticosteroids. _Chronic Graft-versus-host disease_ Jakavi is indicated for the treatment of patients with chronic Graft versus Host Disease (cGvHD) aged 12 years and older who have inadequate response to corticosteroids.
**CONTRAINDICATIONS** Hypersensitivity to the active substance or any of the excipients.
L01EJ01
ruxolitinib
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Novartis Pharma Stein AG
Allpack Group AG (primary and secondary packager - blister packaging)
Active Ingredients
Documents
Package Inserts
Jakavi Tablet PI.pdf
Approved: August 12, 2022
