Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**4.2 Posology and method of administration** **BERODUAL Solution for Inhalation** (1 mL contains 261 mcg ipratropium bromide + 500 mcg fenoterol hydrobromide) (20 drops = 1 mL) Posology Treatment should be initiated and administered under medical supervision, e.g. in the hospital setting. Home based treatment can be recommended in patients when a low dose rapid acting beta-agonist bronchodilator such as BERODUAL metered dose inhaler has been insufficient in providing relief after consultation with an experienced physician. It can also be recommended in patients who are in need for nebuliser treatment for other reasons e.g. handling issues of metered dose inhaler or requirement of higher doses in experienced patients. The treatment with the solution for inhalation should always be started with the lowest recommended dose. The dosage should be adapted to the individual requirements and tailored according to the severity of the acute episode. Administration should be stopped when sufficient symptom relief is achieved. The following dosages are recommended: **Adults (including elderly) and adolescents >12 years of age:** Acute episodes of bronchospasm Depending on the severity of the acute episode doses ranging between 261 mcg ipratropium bromide/500 mcg fenoterol hydrobromide (i.e. 1 mL = 20 drops) and 652.5 mcg ipratropium bromide/1250 mcg fenoterol hydrobromide (i.e. 2.5 mL = 50 drops) may be used. In exceptional particular severe cases doses up to 1044 mcg ipratropium bromide/2000 mcg fenoterol hydrobromide (i.e. 4 mL = 80 drops) may be used. **Children 6 – 12 years:** Acute asthma episodes Depending on the severity of the acute episode and age doses ranging between 130.5 mcg ipratropium bromide/250 mcg fenoterol hydrobromide (i.e. 0.5 mL= 10 drops) and 522 mcg ipratropium bromide/1000 mcg fenoterol hydrobromide (i.e. 2 mL = 40 drops) may be used. **Children < 6 years (below 22 kg body weight):** Because there is limited information in this age group the following dose is recommended to be given under medical supervision only: About 26.1 mcg ipratropium bromide/ 50 mcg fenoterol hydrobromide (i.e. 0.1mL = 2 drops) per kg body weight = up to a maximum of 0.5 mL (=10 drops). Instructions for use The solution for inhalation is intended only for inhalation with suitable nebulising devices and must not be taken orally. The recommended dose is to be diluted with physiological saline to a final volume of 3 – 4 mL and nebulised and inhaled until sufficient symptom relief is achieved. BERODUAL solution for inhalation may, however, not be diluted with distilled water. The solution should be freshly diluted each time before use; any residual diluted solution should be discarded. The diluted solution should be inhaled directly after preparation of the solution. The duration of inhalation can be controlled by the dilution volume. BERODUAL solution for inhalation can be administered using a range of commercially available nebulising devices. The lung and systemic drug exposure is dependent on the nebuliser used and may be higher than with BERODUAL metered dose aerosol depending on the efficiency of the device. Where wall oxygen is available the solution is best administered at a flow rate of 6 – 8 litres per minute. The instructions provided by the manufacturer of the nebulising device for proper care, maintenance and cleaning of the equipment should be followed. **BERODUAL N Metered Dose Inhaler** Posology The dosage should be adapted to the individual requirements. The following dosages are recommended for adults and children > 6 years: **Acute asthma episodes** **21 mcg** ipratropium bromide + **50 mcg** fenoterol hydrobromide 2 actuations are sufficient for prompt symptom relief in many cases. In more severe cases, if breathing has not noticeably improved after 5 minutes, two further actuations may be taken. If an attack has not been relieved by 4 actuations, further actuations may be required. In these cases, patients should be advised to consult the doctor or the nearest hospital immediately. **Intermittent and long-term treatment** (in asthma BERODUAL metered dose aerosol should be used only on an as-needed basis) 1 – 2 actuations for each administration, up to a maximum of 8 actuations per day (average 1 – 2 actuations 3 times daily). In children BERODUAL metered dose aerosol should only be used on medical advice and under the supervision of an adult. Patients should be instructed on the correct administration of the metered dose aerosol to ensure successful therapy (see Instructions for use). Instruction for use: **Before first time use of the metered dose aerosol, the following rules should be observed:** Remove protective cap and depress the valve twice. **Before each use of the metered dose aerosol, the following rules should be observed:** 1. Remove protective cap. (If the inhaler has not been used for more than three days the valve has to be actuated once) 2. Breathe out deeply. 3. Hold the inhaler as shown in fig. 1, and close lips around the mouthpiece. The arrow and the base of the container should be pointing upwards. 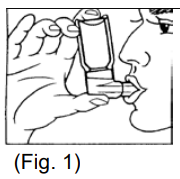 4. Breathe in as deeply as possible, pressing the base of the canister firmly at the same time, this releases one metered dose. Hold the breath for a few seconds, then remove the mouthpiece from the mouth and breathe out. The same action should be repeated for a second inhalation. 5. Replace the protective cap after use. The container is not transparent. It is therefore not possible to see when it is empty. The inhaler will deliver 200\* puffs. When the labelled number of doses have been used the canister may still appear to contain a small amount of fluid. The inhaler should, however, be replaced so that you can be certain that you are getting the right amount of your medicine in each actuation. Clean your mouthpiece at least once a week. It is important to keep the mouthpiece of your inhaler clean to ensure that medicine does not build up and block the spray. For cleaning, first take off the dust cap and remove the canister from the mouthpiece. Rinse warm water through the mouthpiece until no medication build-up and/or dirt is visible. 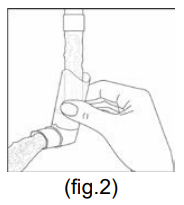 After cleaning shake out the mouthpiece and let it air-dry **without** using any heating system. Once the mouthpiece is dry, replace the canister and the dust cap. 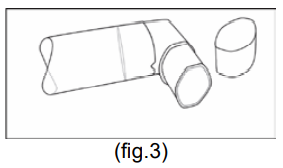 \*300 \[when the inhaler delivers 300 puffs\] WARNING: The plastic mouthpiece has been specially designed for use with BERODUAL metered dose aerosol to ensure that you always get the right amount of the medicine. The mouthpiece must never be used with any other metered aerosol nor must the BERODUAL metered dose aerosol be used with any mouthpiece other than the one supplied with the product. The container is under pressure and should by no account be opened by force or exposed to temperatures above 50°C.
RESPIRATORY (INHALATION)
Medical Information
**4.1 Therapeutic indications** BERODUAL is a bronchodilator for the prevention and treatment of symptoms in chronic obstructive airway disorders with reversible airflow limitation such as bronchial asthma and especially chronic bronchitis with or without emphysema. Concomitant anti-inflammatory therapy should be considered for patients with bronchial asthma and steroid responsive chronic obstructive pulmonary disease (COPD).
**4.3 Contraindications** BERODUAL is contraindicated in patients with known hypersensitivity to fenoterol hydrobromide or atropine-like substances or to any of the excipients of the product. BERODUAL is also contraindicated in patients with hypertrophic obstructive cardiomyopathy and tachyarrhythmia.
R03AK03
xr 03 ak 03
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
ISTITUTO DE ANGELI S.R.L.
Active Ingredients
Documents
Package Inserts
Berodual PI.pdf
Approved: April 21, 2023
