Regulatory Information
SERVIER (S) PTE LTD
SERVIER (S) PTE LTD
Therapeutic
Prescription Only
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Posology The usual posology is one tablet once daily. Patients should be stabilized with bisoprolol and perindopril at the same dose level for at least 4 weeks. The fixed-dose combination is not suitable for initial therapy. For patients stabilized with bisoprolol 2.5 mg and perindopril 2.5 mg: one half 5 mg/5 mg tablet once daily. If a change of posology is required, titration should be done with the individual components. Special population _Renal impairment (see section 4.4 and 5.2_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_ In patients with renal impairment, the recommended dose of Cosyrel 5 mg/5 mg should be based on creatinine clearance as outlined in table 1 below: 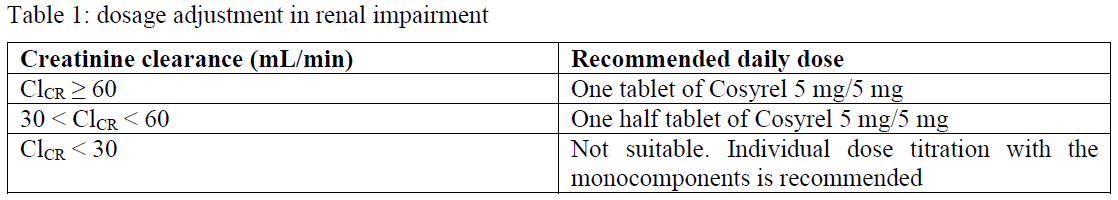 _Hepatic impairment (see section 4.4 and 5.2_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_ No dosage adjustment is necessary in patients with hepatic impairment. _Elderly_ Cosyrel should be administered according to the renal function. _Paediatric population_ The safety and efficacy of Cosyrel in children and adolescents have not been established. No data are available. Therefore, use in children and adolescents is not recommended. Method of administration Cosyrel tablet should be taken as a single dose once daily in the morning before a meal.
ORAL
Medical Information
**4.1 Therapeutic indications** Cosyrel 5 mg/5 mg and Cosyrel 10 mg/5 mg Cosyrel 5 mg/5 mg and Cosyrel 10 mg/5 mg are indicated as substitution therapy for treatment of hypertension and/or stable coronary artery disease (in patients with a history of myocardial infarction and/or revascularisation) and/or stable chronic heart failure with reduced systolic left ventricular function in adult patients adequately controlled with bisoprolol and perindopril given concurrently at the same dose level. Cosyrel 5 mg/10 mg and Cosyrel 10 mg/10 mg Cosyrel 5 mg/10 mg and Cosyrel 10 mg/10 mg are indicated as substitution therapy for treatment of hypertension and/or stable coronary artery disease (in patients with a history of myocardial infarction and/or revascularisation) in adult patients adequately controlled with bisoprolol and perindopril given concurrently at the same dose level.
**4.3 Contraindications** - Hypersensitivity to the active substances, or to any of the excipients listed in section 6.1, or to any other angiotensin converting enzyme (ACE) inhibitor – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - Acute heart failure or during episodes of heart failure decompensation requiring _i.v._ inotropic therapy - Cardiogenic shock - Second or third degree AV block (without pacemaker) - Sick sinus syndrome - Sinoatrial block - Symptomatic bradycardia - Symptomatic hypotension - Severe bronchial asthma or severe chronic obstructive pulmonary disease - Severe forms of peripheral arterial occlusive disease or severe forms of Raynaud's syndrome - Untreated phaeochromocytoma (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - Metabolic acidosis - History of angioedema associated with previous ACE inhibitor therapy - Hereditary or idiopathic angioedema - Second and third trimesters of pregnancy (see sections 4.4 and 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - Concomitant use of Cosyrel with aliskiren-containing products in patients with diabetes mellitus or renal impairment (GFR < 60 ml/min/1.73m2) (see sections 4.4, 4.5 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Concomitant use with sacubitril/valsartan therapy, Cosyrel must not be initiated earlier than 36 hours after the last dose of sacubitril/valsartan (see sections 4.4 and 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), - Extracorporeal treatments leading to contact of blood with negatively charged surfaces (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), - Significant bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
C09BX02
perindopril and bisoprolol
Manufacturer Information
SERVIER (S) PTE LTD
Les Laboratoires Servier Industrie [LSI]
Active Ingredients
Documents
Package Inserts
Cosyrel Film Coated Tablets PI.pdf
Approved: June 21, 2022
