Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** Pharmaceutical form: Film-coated tablets Dosage depends on the age and renal function of the patient and the severity of the infection. To minimise potential gastrointestinal intolerance, administer at the start of a meal. The absorption of Co-amoxiclav Tablets 875-125 mg is optimised when taken at the start of a meal. Treatment should not be extended beyond 14 days without review. Therapy can be started parenterally and continued with an oral preparation. Tablets should be swallowed whole without chewing. If required, tablets may be broken in half and swallowed without chewing. Co-amoxiclav Tablets 875-125 mg tablets are not recommended in children of 12 years and under. **Adults and Children over 12 years** The usual recommended daily dosage is:  **Renal Impairment** No adjustment in dose is required in patients with creatinine clearance (CrCl) greater than 30 mL/min. The _Co-amoxiclav Tablets 875-125 mg_ tablet should only be used in patients with a creatinine clearance (CrCl) rate of more than 30 mL/min. 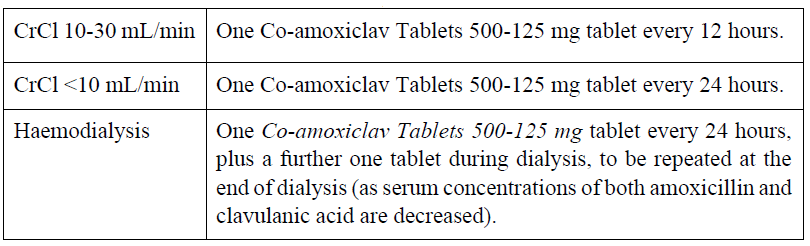 **Hepatic Impairment** Dose with caution; monitor hepatic function at regular intervals.
ORAL
Medical Information
**Indications** Co-amoxiclav Tablets 875-125 mg is an antibiotic agent with a notably broad spectrum of activity against the commonly occurring bacterial pathogens in general practice and hospital. The beta-lactamase inhibitory action of clavulanate extends the spectrum of amoxicillin to embrace a wider range of organisms, including many resistant to other beta-lactam antibiotics. Co-amoxiclav Tablets 875-125 mg should be used in accordance with local official antibiotic-prescribing guidelines and local susceptibility data. Co-amoxiclav Tablets 875-125 mg oral presentations for twice daily dosing, are indicated for short-term treatment of bacterial infections at the following sites: _Upper respiratory tract infections (including ENT)_ e.g. recurrent tonsillitis, sinusitis, otitis media. _Lower respiratory tract infections_ e.g. acute exacerbation of chronic bronchitis (AECB), lobar and bronchopneumonia. _Genito-urinary tract infections_ e.g. cystitis, urethritis, pyelonephritis. _Skin and soft tissue infections_ e.g. boils, abscesses, cellulitis, wound infections. _Bone and joint infections_ e.g. osteomyelitis. _Dental infections_ e.g. dentoalveolar abscess, pericoronitis, acute periodontitis. _Other infections_ e.g. septic abortion, puerperal sepsis, intra-abdominal sepsis. Susceptibility to _Co-amoxiclav_ will vary with geography and time (see _Pharmacological Properties, Pharmacodynamics_ for further information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Local susceptibility data should be consulted where available, and microbiological sampling and susceptibility testing performed where necessary.
**Contraindications** Co-amoxiclav Tablets 875-125 mg is contraindicated in patients with a history of hypersensitivity to betalactams, e.g. penicillins and cephalosporins. Co-amoxiclav Tablets 875-125 mg is contraindicated in patients with a previous history of Co-amoxiclav Tablets 875-125 mg associated jaundice/hepatic dysfunction.
J01CR02
amoxicillin and beta-lactamase inhibitor
Manufacturer Information
APOTHECA MARKETING PTE LTD
AUROBINDO PHARMA LIMITED - UNIT XII
Active Ingredients
Documents
Package Inserts
KOACT 1000 Tablets Package Insert.pdf
Approved: January 11, 2023
