Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SUSPENSION
**4.2 Posology and method of administration** Azacitidine treatment should be initiated and monitored under the supervision of a physician experienced in the use of chemotherapeutic agents. Patients should be premedicated with anti-emetics for nausea and vomiting. Posology The recommended starting dose for the first treatment cycle, for all patients regardless of baseline haematology laboratory values, is 75 mg/m2 of body surface area, injected subcutaneously, daily for 7 days, followed by a rest period of 21 days (28-day treatment cycle). It is recommended that patients be treated for a minimum of 6 cycles. Treatment should be continued as long as the patient continues to benefit or until disease progression. Patients should be monitored for haematologic response/toxicity and renal toxicities (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_); a delay in starting the next cycle or a dose reduction as described below may be necessary. _Laboratory tests_ Liver function tests, serum creatinine and serum bicarbonate should be determined prior to initiation of therapy and prior to each treatment cycle. Complete blood counts should be performed prior to initiation of therapy and as needed to monitor response and toxicity, but at a minimum, prior to each treatment cycle. _Dose adjustment due to haematological toxicity_ Haematological toxicity is defined as the lowest count reached in each cycle (nadir) if platelets ≤ 50.0 × 109/l and/or absolute neutrophil count (ANC) ≤ 1 × 109/l. Recovery is defined as an increase of cell line(s) where haematological toxicity was observed of at least half of the difference of nadir and the baseline count plus the nadir count (i.e. blood count at recovery ≥ nadir count + (0.5 × \[baseline count – nadir count\]). _Patients without reduced baseline blood counts (i.e. White Blood Cells (WBC) ≥ 3.0 × 109/l and ANC ≥ 1.5× 109/l, and platelets ≥ 75.0 × 109/l) prior to the first treatment_ If haematological toxicity is observed following Azacitidine treatment, the next cycle of the therapy should be delayed until the platelet count and the ANC have recovered. If recovery is achieved within 14 days, no dose adjustment is necessary. However, if recovery has not been achieved within 14 days, the dose should be reduced per the following table. Following dose modifications, the cycle duration should return to 28 days. 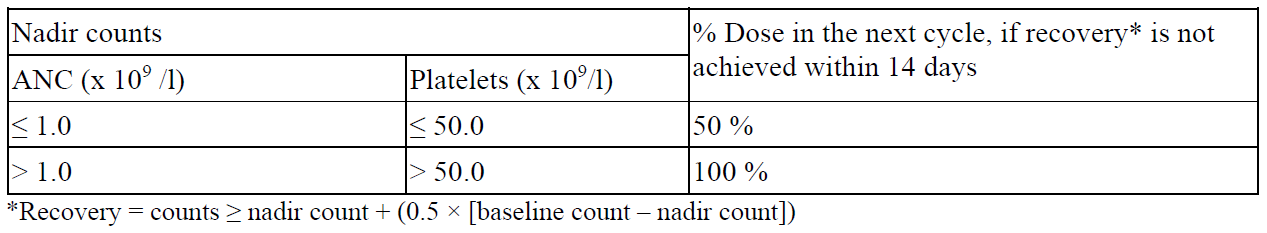 _Patients with reduced baseline blood counts (i.e. WBC < 3.0 × 109/l or ANC < 1.5 × 109/l or platelets < 75.0 x 109/l) prior to the first treatment_ Following Azacitidine treatment, if the decrease in WBC or ANC or platelets from that prior to treatment is ≤ 50 %, or greater than 50 % but with an improvement in any cell line differentiation, the next cycle should not be delayed and no dose adjustment made. If the decrease in WBC or ANC or platelets is greater than 50 % from that prior to treatment, with no improvement in cell line differentiation, the next cycle of Azacitidine therapy should be delayed until the platelet count and the ANC have recovered. If recovery is achieved within 14 days, no dose adjustment is necessary. However, if recovery has not been achieved within 14 days, bone marrow cellularity should be determined. If the bone marrow cellularity is > 50 %, no dose adjustments should be made. If bone marrow cellularity is ≤ 50 %, treatment should be delayed and the dose reduced per the following table: 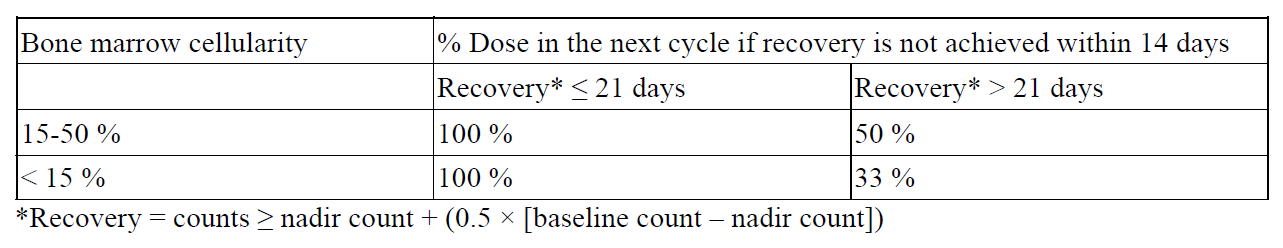 Following dose modifications, the cycle duration should return to 28 days. _Special populations_ _Elderly patients_ No specific dose adjustments are recommended for the elderly. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function. _Patients with renal impairment_ Azacitidine can be administered to patients with renal impairment without initial dose adjustment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If unexplained reductions in serum bicarbonate levels to less than 20 mmol/l occur, the dose should be reduced by 50 % on the next cycle. If unexplained elevations in serum creatinine or blood urea nitrogen (BUN) to ≥ 2-fold above baseline values and above upper limit of normal (ULN) occur, the next cycle should be delayed until values return to normal or baseline and the dose should be reduced by 50 % on the next treatment cycle (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients with hepatic impairment_ No formal studies have been conducted in patients with hepatic impairment (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with severe hepatic organ impairment should be carefully monitored for adverse events. No specific modification to the starting dose is recommended for patients with hepatic impairment prior to starting treatment; subsequent dose modifications should be based on haematology laboratory values. Azacitidine is contraindicated in patients with advanced malignant hepatic tumours (see sections 4.3 and 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The safety and efficacy of Azacitidine in children aged 0–17 years have not yet been established. No data are available. Method of administration Reconstituted Azacitidine should be injected subcutaneously into the upper arm, thigh or abdomen. Injection sites should be rotated. New injections should be given at least 2.5 cm from the previous site and never into areas where the site is tender, bruised, red, or hardened. After reconstitution, the suspension should not be filtered. For instructions on reconstitution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Azacitidine is indicated for the treatment of adult patients who are not eligible for haematopoietic stem cell transplantation (HSCT) with: - intermediate-2 and high-risk myelodysplastic syndromes (MDS) according to the International Prognostic Scoring System (IPSS), - chronic myelomonocytic leukaemia (CMML) with 10–29 % marrow blasts without myeloproliferative disorder, - acute myeloid leukaemia (AML) with 20–30 % blasts and multi-lineage dysplasia, according to World Health Organisation (WHO) classification, Azacitidine is indicated for the treatment of adult patients aged 65 years or older who are not eligible for HSCT with AML with >30% marrow blasts according to the WHO classification.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Advanced malignant hepatic tumours (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Breast-feeding (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01BC07
azacitidine
Manufacturer Information
ADVAGEN PTE. LTD.
Sichuan Huiyu Pharmaceutical Co., Ltd.
Active Ingredients
Documents
Package Inserts
Azacitidine Advagen Powder for Suspension for Injection_PI.pdf
Approved: April 28, 2023
