Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**2\. DOSAGE AND ADMINISTRATION** **2.1 Schizophrenia** **Adults** _Usual Dose_ The recommended starting and target dose for ABILIFY is 10 or 15 mg/day administered on a once-a-day schedule without regard to meals. ABILIFY has been systematically evaluated and shown to be effective in a dose range of 10 to 30 mg/day; however, doses higher than 10 or 15 mg/day were not more effective than 10 or 15 mg/day. Dosage increases should generally not be made before 2 weeks, the time needed to achieve steady state _\[see CLINICAL STUDIES (13.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. _Maintenance Therapy_ While there is no body of evidence available to answer the question of how long a patient treated with aripiprazole should remain on it, systematic evaluation of patients with schizophrenia who had been symptomatically stable on other antipsychotic medication, for periods of 3 months or longer, were discontinued from those medications, and were then administered ABILIFY 15 mg/day and observed for relapse during a period of up to 26 weeks, demonstrated a benefit of such maintenance treatment _\[see CLINICAL STUDIES (13.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Patients should be periodically reassessed to determine the need for maintenance treatment. **Adolescents** _Usual Dose_ The recommended target dose of ABILIFY is 10 mg/day. Aripiprazole was studied in adolescent patients 13 to 17 years of age with Schizophrenia at daily doses of 10 mg and 30mg. The starting daily dose of the tablet formulation in these patients was 2 mg, which was titrated to 5 mg after 2 days and to the target dose of 10 mg after 2 additional days. Subsequent dose increases should be administered in 5 mg increments. The 30 mg/day dose was not shown to be more efficacious than the 10 mg/day dose and was associated with a higher incidence of significant adverse reactions including extrapyramidal disorder, somnolence and tremor _\[see ADVERSE REACTIONS (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. ABILIFY can be administered without regard to meals _\[see CLINICAL STUDIES (13.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. _Maintenance Therapy_ The efficacy of ABILIFY for the maintenance treatment of schizophrenia in the adolescent population has not been evaluated. While there is no body of evidence available to answer the question of how long the adolescent patient treated with ABILIFY should be maintained on the drug, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and paediatric patients. Thus, it is generally recommended that responding patients be continued beyond the acute response, but at the lowest dose needed to maintain remission. Patients should be periodically reassessed to determine the need for maintenance treatment. **Switching from Other Antipsychotics** There are no systematically collected data to specifically address switching patients with schizophrenia from other antipsychotics to ABILIFY or concerning concomitant administration with other antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized. **2.2 Bipolar Disorder** _Acute Treatment of Manic and Mixed Episodes_ **Adults** The recommended starting dose in adults is 15 mg given once daily as monotherapy and 10 to 15 mg given once daily as adjunctive therapy with lithium or valproate. ABILIFY can be given without regard to meals. The recommended target dose of ABILIFY is 15 mg/day, as monotherapy or as adjunctive therapy with lithium or valproate. The dose may be increased to 30 mg/day based on clinical response. The safety of doses above 30 mg/day has not been evaluated in clinical trials. **Adolescents** The recommended starting dose in adolescent patients as monotherapy is 2 mg/day, with titration to 5 mg/day after 2 days, and a target dose of 10 mg/day after 2 additional days. Subsequent dose increases, if needed, should be administered in 5 mg/day increments. ABILIFY can be given without regard to meals. Enhanced efficacy at doses higher than a daily dose of 10 mg has not been demonstrated, and a daily dose of 30 mg is associated with a substantially higher incidence of significant adverse reactions including extrapyramidal disorder, somnolence, akathisia and salivary hypersecretion. Doses higher than 10 mg/day should therefore only be used in exceptional cases and with close clinical monitoring _\[see WARNINGS AND PRECAUTIONS (4.6), ADVERSE REACTIONS (5.1), and CLINICAL STUDIES (13.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Younger patients are at increased risk of experiencing adverse events associated with aripiprazole. Therefore, ABILIFY is not recommended for use in patients below 13 years of age _\[see ADVERSE REACTIONS (5.1) and CLINICAL STUDIES (13.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. _Maintenance Therapy_ The recommended dose for maintenance treatment is the same dose needed to stabilize patients during acute treatment, both for adult and paediatric patients. Systematic evaluation of adult patients with Bipolar I Disorder experiencing a manic or mixed episode, who had been symptomatically stable on ABILIFY Tablets (15 mg/day or 30 mg/day with a starting dose of 30 mg/day) for 6 consecutive weeks and then randomized to ABILIFY Tablets (15 mg/day or 30 mg/day) or placebo for at least 6 months and up to an additional 17 months of observation for relapse, demonstrated a benefit of such maintenance treatment _\[see CLINICAL STUDIES (13.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Patients should be periodically reassessed to determine the need for maintenance treatment. **2.3 Adjunctive Treatment of Major Depressive Disorder** The recommended starting dose for ABILIFY as adjunctive treatment for patients already taking an antidepressant is 2 to 5 mg/day. The recommended dosage range is 2 to 15 mg/day. Dosage adjustments of up to 5 mg/day should occur gradually, at intervals of no less than 1 week _\[see CLINICAL STUDIES (13.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Patients should be periodically reassessed to determine the continued need for maintenance treatment. **2.4 Irritability Associated with Autistic Disorder** **Paediatric Patients (6 to 17 years)** The recommended dosage range for the treatment of paediatric patients with irritability associated with autistic disorder is 5 to 15 mg/day. Dosing should be initiated at 2 mg/day. The dose should be increased to 5 mg/day, with subsequent increases to 10 or 15 mg/day if needed. Dose adjustments of up to 5 mg/day should occur gradually, at intervals of no less than 1 week _\[see CLINICAL STUDIES (13.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Patients should be periodically reassessed to determine the continued need for maintenance treatment. **2.5 Tourette’s Disorder** **Paediatric Patients (6 to 18 years)** The recommended dosage range for Tourette’s Disorder is 5 to 20 mg/day. For patients weighing less than 50 kg, dosing should be initiated at 2 mg/day with a target dose of 5 mg/day after 2 days. The dose can be increased to 10 mg/day in patients who do not achieve optimal control of tics. Dosage adjustments should occur gradually at intervals of no less than 1 week. For patients weighing 50 kg or more, dosing should be initiated at 2 mg/day for 2 days, and then increased to 5 mg/day for 5 days, with a target dose of 10 mg/day on day 8. The dose can be increased up to 20 mg/day for patients who do not achieve optimal control of tics. Dosage adjustments should occur gradually in increments of 5 mg/day at intervals of no less than 1 week _\[see CLINICAL STUDIES (13.5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Patients should be periodically reassessed to determine the continued need for maintenance treatment. **2.6 Dosage Adjustments for Cytochrome P450 Considerations** Dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in patients taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers (see Table 1). When the co-administered drug is withdrawn from the combination therapy, ABILIFY dosage should then be adjusted to its original level. When the co-administered CYP3A4 inducer is withdrawn, ABILIFY dosage should be reduced to the original level over 1 to 2 weeks. Patients who may be receiving a combination of strong, moderate, and weak inhibitors of CYP3A4 and CYP2D6 (e.g., a strong CYP3A4 inhibitor and a moderate CYP2D6 inhibitor or a moderate CYP3A4 inhibitor with a moderate CYP2D6 inhibitor), the dosing may be reduced to one-quarter (25%) of the usual dose initially and then adjusted to achieve a favourable clinical response. 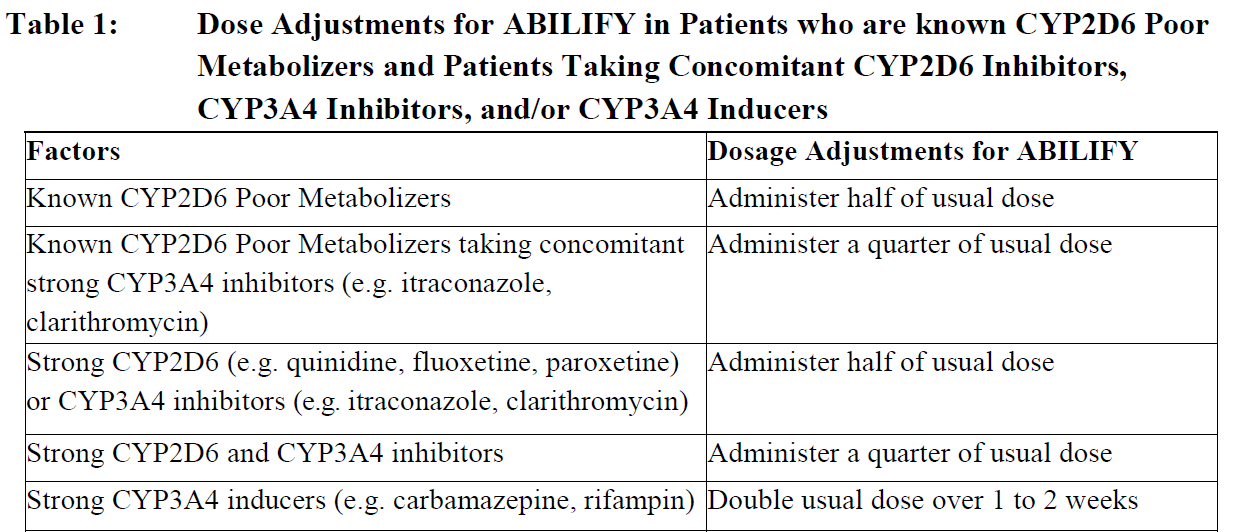 When adjunctive ABILIFY is administered to patients with major depressive disorder, ABILIFY should be administered without dosage adjustment as specified in _DOSAGE AND ADMINISTRATION (2.3)_. **2.7 Dosing of Oral Solution** The oral solution can be substituted for tablets on an mg-per-mg basis up to the 25 mg dose level. Patients receiving 30 mg tablets should receive 25 mg of the solution _\[see CLINICAL PHARMACOLOGY (11.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
ORAL
Medical Information
**1\. INDICATIONS AND USAGE** **Schizophrenia** ABILIFY is indicated for the treatment of schizophrenia. The efficacy of ABILIFY in the treatment of schizophrenia was established in four short-term (4- and 6-week) controlled trials in adults and one 6-week trial in paediatrics (13 to 17 years). Maintenance efficacy was demonstrated in one trial in adults and can be extrapolated to paediatrics _\[see CLINICAL STUDIES (13.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. The physician who elects to use ABILIFY for extended periods should periodically re-evaluate the long- term usefulness of the drug for the individual patient _\[see DOSAGE AND ADMINISTRATION (2)\]_. **Bipolar I Disorder** ABILIFY is indicated for the treatment of acute manic and mixed episodes associated with Bipolar I Disorder and for maintaining stability or preventing recurrence, as monotherapy in adults and in adolescents aged 13 years and older, and as an adjunct to lithium or valproate in adults. The efficacy of ABILIFY as monotherapy was established in four 3-week monotherapy trials in adults and one 4-week monotherapy trial in paediatric patients. Efficacy as adjunctive therapy was established in one 6-week adjunctive trial in adults _\[see CLINICAL STUDIES (13.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Maintenance efficacy was demonstrated in one monotherapy maintenance trial and in one adjunctive maintenance trial in adults _\[see CLINICAL STUDIES (13.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Physicians who elect to use ABILIFY for extended periods, should periodically re-evaluate the long-term usefulness of the drug for the individual patient _\[see DOSAGE AND ADMINISTRATION (2)\]_. **Adjunctive Treatment of Major Depressive Disorder** ABILIFY is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD). Efficacy was established in three 6-week trials in adults with MDD who had an inadequate response to antidepressant therapy during the current episode _\[see CLINICAL STUDIES (13.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **Irritability Associated with Autistic Disorder** ABILIFY is indicated for the treatment of irritability associated with autistic disorder. Efficacy was established in two 8-week trials in paediatric patients (aged 6 to 17 years) with irritability associated with autistic disorder (including symptoms of aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods) _\[see CLINICAL STUDIES (13.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. The efficacy of ABILIFY for the maintenance treatment of irritability associated with autistic disorder was not established. **Tourette’s Disorder** ABILIFY is indicated for the treatment of Tourette’s disorder. Efficacy was established in one 8-week (aged 7 to 17 years) and one 10-week (aged 6 to 18 years) placebo-controlled trial in paediatric patients with Tourette’s disorder _\[see CLINICAL STUDIES (13.5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
**3\. CONTRAINDICATIONS** ABILIFY is contraindicated in patients with a history of a hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis.
N05AX12
aripiprazole
Manufacturer Information
OTSUKA PHARMACEUTICALS (SINGAPORE) PTE. LTD.
OTSUKA PHARMACEUTICAL CO. LTD.
Bristol-Myers Squibb Manufacturing Company {Humacao]
Abdi Ibrahim IIaç San. Ve Tic. A.Ş.
Active Ingredients
Documents
Package Inserts
Abilify PI.pdf
Approved: August 25, 2022
