Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Treatment with Spinraza should only be initiated by a physician with experience in the management of spinal muscular atrophy (SMA). The decision to treat should be based on an individualised expert evaluation of the expected benefits of treatment for that individual, balanced against the potential risk of treatment with Spinraza. Patients with profound hypotonia and respiratory failure at birth, where Spinraza has not been studied, may not experience a clinically meaningful benefit due to severe survival motor neuron \[SMN\] protein deficiency. Posology The recommended dosage is 12 mg (5 ml) per administration. Spinraza treatment should be initiated as early as possible after diagnosis with 4 loading doses on Days 0, 14, 28 and 63. A maintenance dose should be administered once every 4 months thereafter. _Duration of treatment_ Information on long term efficacy of this medicinal product is not available. The need for continuation of therapy should be reviewed regularly and considered on an individual basis depending on the patient’s clinical presentation and response to the therapy. _Missed or delayed doses_ If a loading or a maintenance dose is delayed or missed, Spinraza should be administered according to the schedule in Table 1 below. 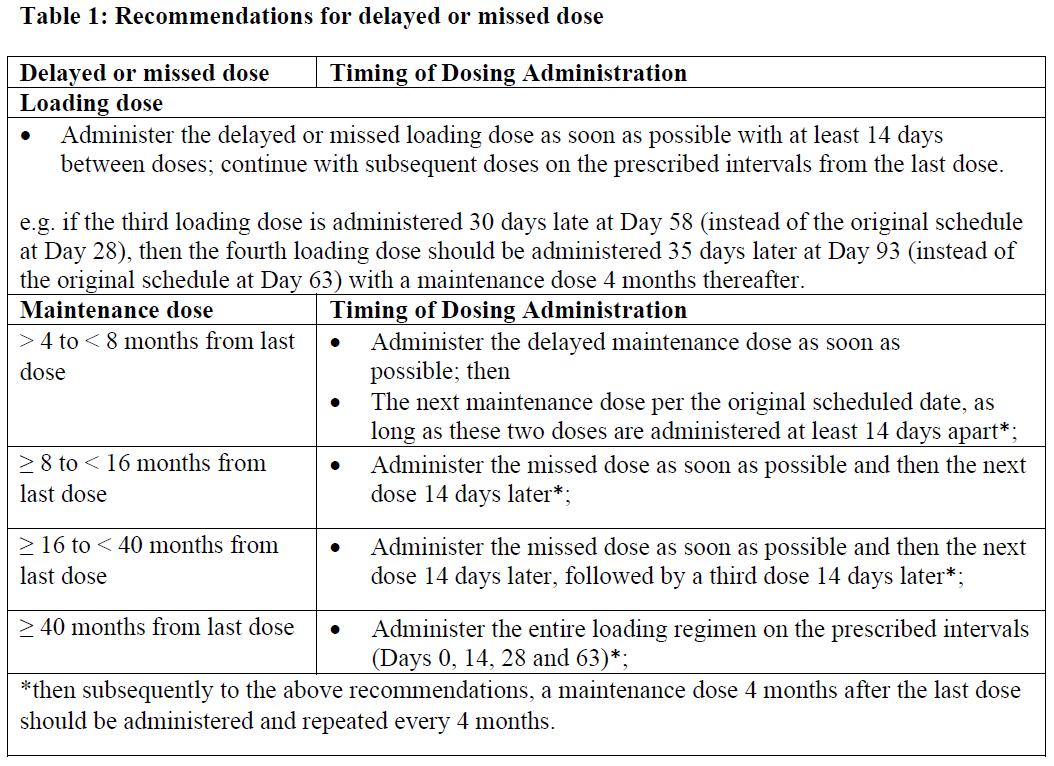 Special populations _Renal impairment_ Nusinersen has not been studied in patients with renal impairment. The safety and efficacy in patients with renal impairment has not been established and they should be closely observed. _Hepatic impairment_ Nusinersen has not been studied in patients with hepatic impairment. Nusinersen is not metabolised via the cytochrome P450 enzyme system in the liver, therefore dose adjustment is unlikely to be required in patients with hepatic impairment (see sections 4.5 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration Spinraza is for intrathecal use by lumbar puncture. Treatment should be administered by health care professionals experienced in performing lumbar punctures. Spinraza is administered as an intrathecal bolus injection over 1 to 3 minutes, using a spinal anaesthesia needle. The injection must not be administered in areas of the skin where there are signs of infection or inflammation. It is recommended that the volume of cerebral spinal fluid (CSF), equivalent to the volume of Spinraza to be injected, is removed prior to administration of Spinraza. Sedation may be required to administer Spinraza, as indicated by the clinical condition of the patient. Ultrasound (or other imaging techniques) may be considered to guide intrathecal administration of Spinraza, particularly in younger patients and in patients with scoliosis ; see instructions for use in section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRATHECAL
Medical Information
**4.1 Therapeutic indications** Spinraza is indicated for the treatment of 5q Spinal Muscular Atrophy.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
M09AX07
nusinersen
Manufacturer Information
ZUELLIG PHARMA PTE. LTD.
Patheon Italia S.p.A.
Vetter Pharma-Fertigung GmbH & Co. KG
Active Ingredients
Documents
Package Inserts
Spinraza Solution for Injection PI.pdf
Approved: March 31, 2023
