Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
LOZENGE
**HOW TO USE THIS MEDICINE** 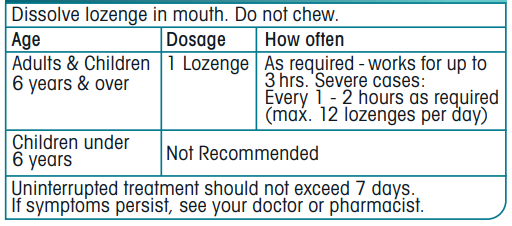
ORAL
Medical Information
**USE THIS MEDICINE FOR** Fast relief from the symptoms of: - Sore throat - Pharyngitis - Tonsillitis - Mouth ulcers - Swelling, redness and inflammatory conditions - Pain following mouth or throat surgery - Pain following dental procedures
**DO NOT USE THIS MEDICINE IF** You are allergic to benzydamine or other anti-inflammatory medicines. If an allergic reaction occurs, stop taking and see your doctor immediately.
A01AD02
benzydamine
Manufacturer Information
INOVA PHARMACEUTICALS (SINGAPORE) PTE. LIMITED
Unique Pharmaceuticals Laboratories
Active Ingredients
Documents
Patient Information Leaflets
Difflam lozenges Eucalyptus Menthol Outer Carton.pdf
Approved: March 12, 2019
