Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**DOSAGE AND ADMINISTRATION** Consideration should be given to official/national guidance on the appropriate use of antifungal agents. Treatment with Mycamine should be initiated by a physician experienced in the management of fungal infections. Specimens for fungal culture and other relevant laboratory studies (including histopathology) should be obtained prior to therapy to isolate and identify causative organism(s). Therapy may be instituted before the results of the cultures and other laboratory studies are known. However, once these results become available, antifungal therapy should be adjusted accordingly. Mycamine should be administered once daily by intravenous infusion. The dosage depends on the indication and body weight of the patient as shown in Table 8 below. 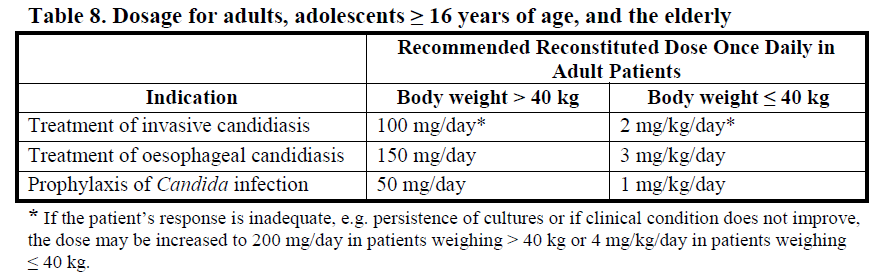 A loading dose is not required. Typically, 85% of the steady-state concentration is achieved after three daily Mycamine doses. Treatment duration _Invasive candidiasis:_ The treatment duration of _Candida_ infection should be a minimum of 14 days. The antifungal treatment should continue for at least one week after two sequential negative blood cultures have been obtained and after resolution of clinical signs and symptoms of infection. _Oesophageal candidiasis:_ For the treatment of oesophageal candidiasis, Mycamine should be administered for at least one week after resolution of clinical signs and symptoms. _Prophylaxis of Candida infections:_ For prophylaxis of _Candida_ infection, Mycamine should be administered for at least one week after neutrophil recovery. 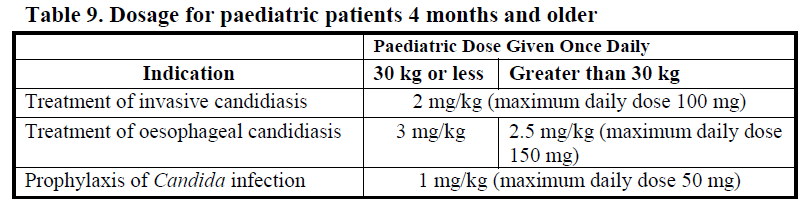 Patients with hepatic impairment No dosage adjustment is required in patients with mild or moderate hepatic impairment (see PHARMACOLOGY, Pharmacokinetic characteristics in special populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There are currently insufficient data available for the use of Mycamine in patients with severe hepatic impairment and its use is not recommended in these patients. Patients with renal impairment No dosage adjustment is required in patients with renal impairment (creatinine clearance < 30mL/min) (see PHARMACOLOGY, Pharmacokinetic characteristics in special populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Instructions for reconstitution and dilution Mycamine must not be mixed or co-infused with any other medicinal products except those mentioned below. Mycamine has been shown to precipitate when mixed directly with a number of other commonly used medications. Using aseptic techniques at room temperature, Mycamine should be reconstituted and diluted as follows: 1. Remove the plastic cap from the vial and disinfect the stopper with alcohol. 2. Five mL of sodium chloride 9 mg/mL (0.9%) solution for infusion or glucose 50 mg/mL (5%) solution for infusion (taken from a 100 mL bag/bottle) should be aseptically and slowly injected into each vial along the side of the inner wall. Although the concentrate will foam, every effort should be made to minimise the amount of foam generated. A sufficient number of vials of Mycamine should be reconstituted to obtain the required dose as shown in Table 10 below. 3. The vial should be rotated gently. DO NOT SHAKE. The powder will dissolve completely. The concentrate should be used immediately for further dilution. Each vial is for single use only; any unused reconstituted concentrate should be discarded immediately. 4. All of the reconstituted concentrate should be withdrawn from each vial and returned into the infusion bag/bottle from which it was originally taken. The diluted infusion solution should be used immediately. 5. The infusion bag/bottle should be gently inverted to disperse the diluted solution but NOT agitated in order to avoid foaming. Do not use if the solution is cloudy or has precipitated. 6. The infusion bag/bottle containing the diluted infusion solution should be inserted into a closable opaque bag for protection from light. 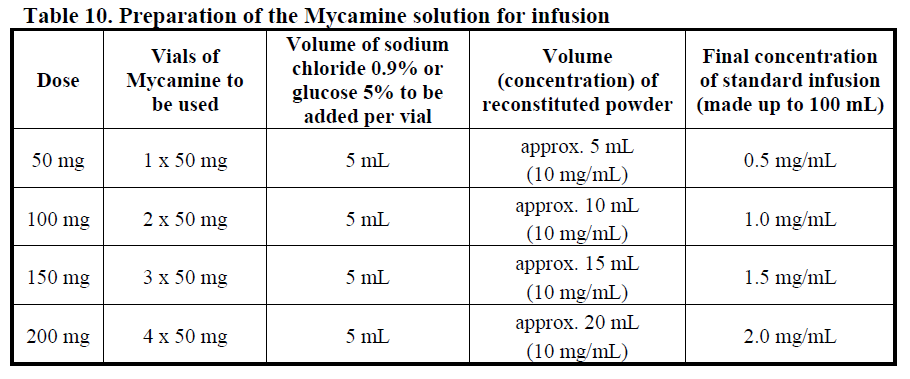 As with all parenteral drug products, reconstituted Mycamine should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use material if there is any evidence of precipitation or foreign matter. Aseptic technique must be strictly observed in all handling since no preservative or bacteriostatic agent is present in Mycamine or in the materials specified for reconstitution and dilution. Administration **An existing intravenous line should be flushed with sodium chloride 0.9% solution prior to infusion of Mycamine.** Administer the reconstituted and diluted Mycamine solution intravenously over approximately one hour. More rapid infusions may result in more frequent histamine mediated reactions. **Paediatric Patients** **Mycamine should be infused over one hour. To minimize the risk of infusion reactions, concentrations of greater than 1.5 mg/mL should be administered via central catheter.**
INTRAVENOUS
Medical Information
**INDICATIONS** Mycamine is indicated for: Adults and paediatric patients 4 months and older for: - treatment of invasive candidiasis - treatment of oesophageal candidiasis in patients for whom intravenous therapy is appropriate - prophylaxis of _Candida_ infection in patients undergoing allogeneic haematopoietic stem cell transplantation or patients who are expected to have neutropenia (absolute neutrophil count < 500 cells/microlitre) for 10 or more days. Mycamine has not been adequately studied in patients with endocarditis, osteomyelitis and meningitis due to _Candida_ infections. The decision to use Mycamine should take into account a potential risk for the development of liver tumours. Mycamine should therefore only be used if other antifungals are not appropriate.
**CONTRAINDICATIONS** Mycamine is contraindicated in patients with hypersensitivity to any component of this medication or to other echinocandins (see DESCRIPTION – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
J02AX05
micafungin
Manufacturer Information
ASTELLAS PHARMA SINGAPORE PTE. LTD.
Astellas Pharma Inc.
PATHEON ITALIA S.P.A. (Bulk manufacturer/ Primary Packager)
Active Ingredients
Documents
Package Inserts
Mycamine Powder for Solution for Infusion PI.pdf.pdf
Approved: September 23, 2022
