Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and Administration** The dosage of RAPIFEN should be individualized according to age, body weight, physical status, underlying pathological condition, use of other drugs and type of surgery and anesthesia. The initial dose should be reduced in the elderly (> 65 years of age) and in debilitated patients. In children it should be increased. The effect of the initial dose should be taken into account in determining supplemental doses. To avoid bradycardia, a small intravenous (I.V.) dose of an anti-cholinergic agent just before anesthetic induction may be administered. **Dosage** _**Adults**_ _**For use as an anesthetic induction agent**_ An I.V. bolus dose of ≥120 mcg/kg (17 mL/70 kg) RAPIFEN will induce unconsciousness and analgesia while maintaining good cardiovascular stability in patients with adequate muscle relaxation. **_For short procedures and use in outpatients_** Small doses of RAPIFEN are most useful for minor, short surgical procedures and for outpatients, provided cardiopulmonary monitoring equipment is available. An I.V. bolus dose of 7 to 15 mcg/kg (1 to 2 mL/70 kg) is usually adequate for procedures lasting less than 10 minutes. Should the duration of the procedure exceed 10 minutes, further increments of 7 to 15 mcg/kg (1 to 2 mL/70 kg) should be given every 10 to 15 minutes or as required. Although ventilatory support must be available, spontaneous breathing is maintained in most instances with a dose of 7 mcg/kg (1 mL/70 kg) or less, slowly injected; suggested increments with this technique are 3.5 mcg/kg (0.5 mL/70 kg). When postoperative nausea occurs, it is of relatively short duration and usually controlled by conventional measures. _**For procedures of medium duration**_ The dose of initial I.V. bolus should be adapted to the expected duration of the surgical procedure as follows: 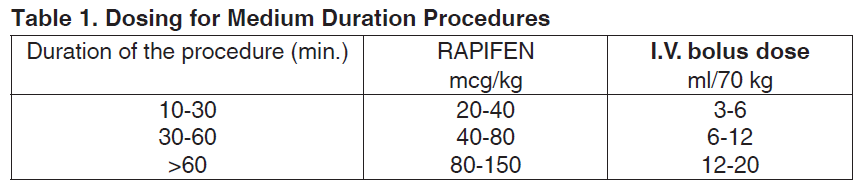 When surgery is more prolonged or more aggressive, analgesia can be maintained by: - either increments of 15 mcg/kg (2 mL/70 kg) RAPIFEN when required (to avoid postoperative respiratory depression, no RAPIFEN should be administered during the last 10 minutes of surgery); - or a RAPIFEN infusion at a rate of 1 mcg/kg/min (0.14 mL/70 kg/min) until 5 to 10 minutes before the completion of surgery. Periods of painful stimuli can easily be managed by small dose increments or by temporarily increasing the infusion rate. When RAPIFEN is used without N2O/O2 or another inhalation anesthetic, a higher maintenance dose of RAPIFEN is required. _**For long procedures**_ RAPIFEN may be used as the analgesic component of anesthesia for long lasting surgical procedures especially when rapid extubation is indicated. Optimum analgesia and stable autonomic condition are maintained through an individually adapted initial intravenous dose and by adjusting the infusion rate to the severity of the surgical stimuli and the reactions of the patient. **Administration** RAPIFEN is administered by bolus injection, or bolus supplemented by increments, or by infusion
INTRAVENOUS
Medical Information
**Indications** RAPIFEN is indicated for use as: - an anesthetic induction agent. - an opioid analgesic in general as well as an adjuvant to regional anesthesia and for both short (bolus injections) and long (bolus, supplemented by increments or by infusion) surgical procedures. Because of its rapid and short-lasting action, RAPIFEN is particularly suited as an opioid analgesic for short procedures and outpatient surgery, but also as an analgesic supplement for procedures of medium and long duration, since periods of very painful stimuli can usually be overcome by small increments of RAPIFEN or by adapting its infusion rate.
**Contraindications** Known intolerance to either of its components or to other opioids.
N01AH02
alfentanil
Manufacturer Information
ALLIANCE PHARM PTE. LTD.
GlaxoSmithKline (GSK) Manufacturing S.p.A.
DEMO SA PHARMACEUTICAL INDUSTRY
Active Ingredients
Documents
Package Inserts
Rapifen_PI.pdf
Approved: March 28, 2023
