Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**Dosage and Administration** As with other neuromuscular blocking agents, monitoring of neuromuscular function is recommended during the use of _NIMBEX_ in order to individualise dosage requirements. - **Use by I.V. bolus injection in adults** **Tracheal intubation**: The recommended intubation dose of _NIMBEX_ for adults is 0.15 mg/kg administered rapidly over 5 to 10 seconds. This dose produces good to excellent conditions for tracheal intubation 120 seconds following injection. Higher doses will shorten the time to onset of neuromuscular block. Table 1 summarises mean pharmacodynamic data when _NIMBEX_ injection was administered at doses of 0.1 to 0.4 mg/kg to healthy adult patients during opioid (thiopentone/fentanyl/midazolam) or propofol anaesthesia. 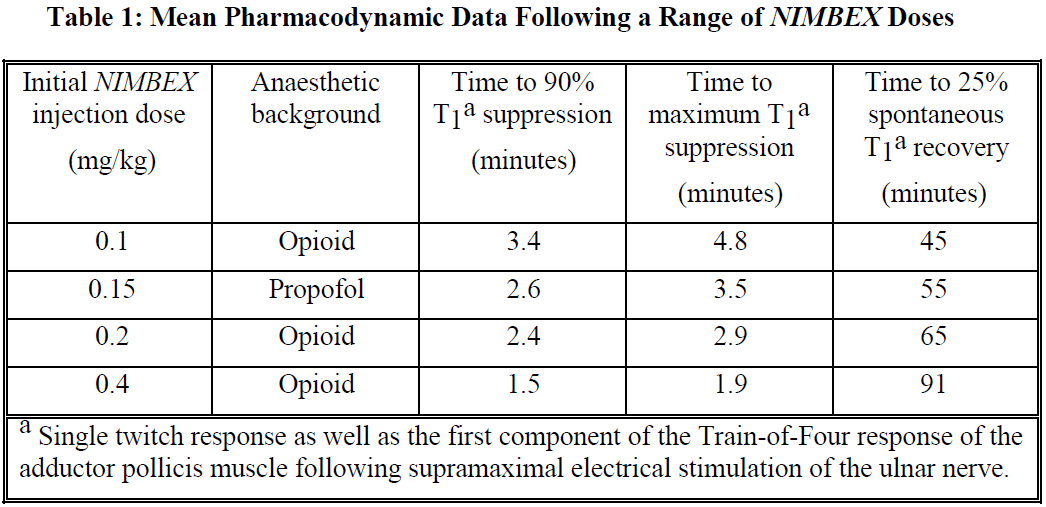 Enflurane or isoflurane anaesthesia may extend the clinically effective duration of an initial dose of _NIMBEX_ by as much as 15%. **Maintenance**: Neuromuscular block can be extended with maintenance doses of _NIMBEX_. A dose of 0.03 mg/kg provides approximately 20 minutes of additional clinically effective neuromuscular block during opioid or propofol anaesthesia. Consecutive maintenance doses do not result in progressive prolongation of effect. **Spontaneous recovery**: Once spontaneous recovery from neuromuscular block is underway, the rate is independent of the _NIMBEX_ dose administered. During opioid or propofol anaesthesia, the median times from 25 to 75% and from 5 to 95% recovery are approximately 13 and 30 minutes, respectively. **Reversal**: Neuromuscular block following _NIMBEX_ administration is readily reversible with standard doses of anticholinesterase agents. The mean times from 25 to 75% recovery and to full clinical recovery (T4:T1 ratio more than or equal to 0.7) are approximately 2 and 5 minutes, respectively, following administration of the reversal agent at an average of 13% T1 recovery. - **Use by I.V. bolus injection in children (1 month to 12 years of age)** _NIMBEX_ has not been studied for intubation in ASA Class III–IV paediatric patients. There are limited data on the use of _NIMBEX_ in paediatric patients under 2 years of age undergoing prolonged or major surgery. **Tracheal intubation**: As in adults, the recommended initial intubation dose of _NIMBEX_ is 0.15 mg/kg administered rapidly over 5 to 10 seconds. This dose produces good to excellent conditions for tracheal intubation 120 seconds following injection of _NIMBEX_. Pharmacodynamic data for this dose are presented in the tables 2 and 3. If a shorter clinical duration is required, pharmacodynamic data suggest that a dose of 0.1 mg/kg may produce similar intubation conditions at 120 to 150 seconds. In paediatric patients aged 1 month to 12 years, _NIMBEX_ has a shorter clinically effective duration and a faster spontaneous recovery profile than those observed in adults under similar anaesthetic conditions. Small differences in the pharmacodynamic profile were observed between the age ranges 1 to 11 months and 1 to 12 years which are summarised in Tables 2 and 3 below. 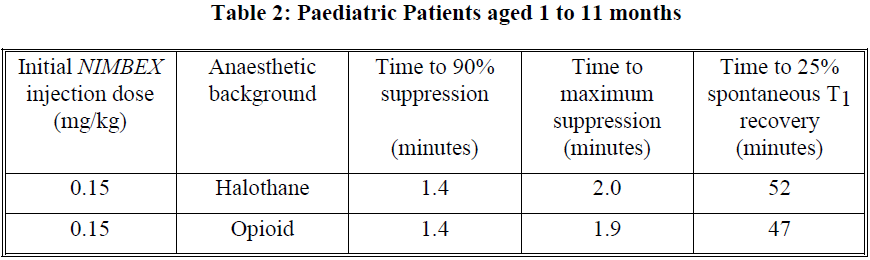 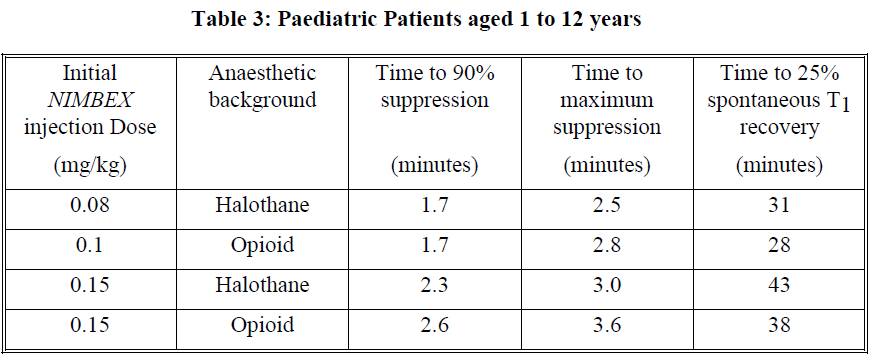 Halothane may be expected to extend the clinically effective duration of _NIMBEX_ by up to 20%. No information is available on the use of _NIMBEX_ in children during isoflurane or enflurane anaesthesia but these agents may also be expected to extend the clinically effective duration of a dose of _NIMBEX_ by up to 20%. **Maintenance (paediatric patients aged 2–12 years)**: Neuromuscular block can be extended with maintenance doses of _NIMBEX_ injection. A dose of 0.02 mg/kg provides approximately 9 minutes of additional clinically effective neuromuscular block during halothane anaesthesia. Consecutive maintenance doses do not result in progressive prolongation of effect. There are insufficient data to make a specific recommendation for maintenance dosing in paediatric patients under 2 years of age. However, very limited data from clinical studies in paediatric patients under 2 years age suggest that a maintenance dose of 0.03 mg/kg may extend clinically effective neuromuscular block for a period of up to 25 minutes during opioid anaesthesia. **Spontaneous recovery**: Once recovery from neuromuscular block is underway, the rate is independent of the _NIMBEX_ dose administered. During opioid or halothane anaesthesia, the median times from 25 to 75% and from 5 to 95% recovery are approximately 11 and 28 minutes, respectively. **Reversal**: Neuromuscular block following _NIMBEX_ administration is readily reversible with standard doses of anticholinesterase agents. The mean times from 25 to 75% recovery and to full clinical recovery (T4:T1 ratio more than or equal to 0.7) are approximately 2 and 5 minutes, respectively, following administration of the reversal agent at an average of 13% T1 recovery. - **Use by I.V. infusion in adults and children (2 to 12 years of age)** Maintenance of neuromuscular block may be achieved by infusion of _NIMBEX_. An initial infusion rate of 3 micrograms/kg/min (0.18 mg/kg/h) is recommended to restore 89 to 99% T1 suppression following evidence of spontaneous recovery. After an initial period of stabilisation of neuromuscular block, a rate of 1 to 2 micrograms/kg/min (0.06 to 0.12 mg/kg/h) should be adequate to maintain block in this range in most patients. Reduction of the infusion rate by up to 40% may be required when _NIMBEX_ is administered during isoflurane or enflurane anaesthesia. ( _see Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The infusion rate will depend upon the concentration of _NIMBEX_ in the infusion solution, the desired degree of neuromuscular block, and the patient's weight. Table 4 provides guidelines for delivery of undiluted _NIMBEX_. 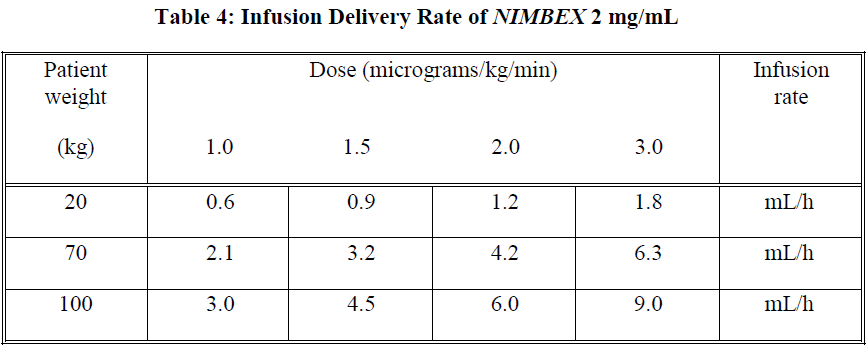 Steady rate continuous infusion of _NIMBEX_ is not associated with a progressive increase or decrease in neuromuscular blocking effect. Following discontinuation of infusion of _NIMBEX_, spontaneous recovery from neuromuscular block proceeds at a rate comparable to that following administration of a single bolus. - **Neonates aged less than 1 month** No dosage recommendation for neonates can be made as administration of _NIMBEX_ has not been studied in this patient population. - **Elderly** No dosing alterations are required in elderly patients. In these patients _NIMBEX_ has a similar pharmacodynamic profile to that observed in young adult patients but, as with other neuromuscular blocking agents, it may have a slightly slower onset. - **Patients with renal impairment** No dosing alterations are required in patients with renal failure. In these patients _NIMBEX_ has a similar pharmacodynamic profile to that observed in patients with normal renal function but it may have a slightly slower onset. - **Patients with hepatic impairment** No dosing alterations are required in patients with end-stage liver disease. In these patients _NIMBEX_ has a similar pharmacodynamic profile to that observed in patients with normal hepatic function but it may have a slightly faster onset. - **Patients with cardiovascular disease** When administered by rapid bolus injection (over 5 to 10 seconds) to patients with serious cardiovascular disease _NIMBEX_ has not been associated with clinically significant cardiovascular effects at any dose studied (up to and including 0.4 mg/kg (8 x ED95)). However, there are limited data for doses above 0.3 mg/kg in this patient population. _NIMBEX_ has not been studied in children undergoing cardiac surgery. - **ICU patients** _NIMBEX_ may be administered by bolus dose and/or infusion to adult patients in the ICU. An initial infusion rate of _NIMBEX_ of 3 micrograms/kg/min (0.18 mg/kg/h) is recommended for adult ICU patients. There may be wide inter-patient variation in dosage requirements and these may increase or decrease with time. In clinical studies the average infusion rate was 3 micrograms/kg/min \[range 0.5 to 10.2 micrograms/kg/min (0.03 to 0.6 mg/kg/h)\]. Table 5 provides guidelines for delivery of undiluted _NIMBEX_. The median time to full spontaneous recovery following long-term (up to 6 days) infusion of _NIMBEX_ in ICU patients was approximately 50 minutes.  The recovery profile after infusions of _NIMBEX_ to ICU patients is independent of duration of infusion. - **Patients undergoing hypothermic cardiac surgery** There have been no studies of _NIMBEX_ in patients undergoing surgery with induced hypothermia (25°C to 28°C). As with other neuromuscular blocking agents, the rate of infusion required to maintain adequate surgical relaxation under these conditions may be expected to be significantly reduced.
INTRAVENOUS
Medical Information
**Indications** _NIMBEX_ is an intermediate-duration, non-depolarising neuromuscular blocking agent for intravenous (i.v.) administration. _NIMBEX_ is indicated for use during surgical and other procedures and in intensive care. It is used as an adjunct to general anaesthesia, or sedation in the Intensive Care Unit (ICU), to relax skeletal muscles, and to facilitate tracheal intubation and mechanical ventilation. _NIMBEX_ contains no antimicrobial preservative and is intended for single patient use.
**Contraindications** _NIMBEX_ is contraindicated in patients known to be hypersensitive to cisatracurium, atracurium, or benzenesulfonic acid.
M03AC11
cisatracurium
Manufacturer Information
DCH AURIGA SINGAPORE
GlaxoSmithKline Manufacturing S.p.A.
Active Ingredients
Documents
Package Inserts
Nimbex PI.pdf
Approved: February 27, 2018
