Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** **Adults** The dosage and duration of therapy shall be established depending on type and severity of infection and the condition of the patient. The recommended daily dosage is as follows: 500 mg IV every 8 hours in the treatment of pneumonia, UTI, gynaecological infections such as endometritis, skin and skin structure infections. 1 g IV every 8 hours in the treatment of nosocomial pneumonias, peritonitis, presumed infections in neutropenic patients, septicaemia. In meningitis the recommended dosage is 2 g every 8 hours. When treating infections known or suspected to be caused by Pseudomonas aeruginosa, a dose of at least 1g every 8 hours in adults (maximum approved dose is 6g daily given in 3 divided doses) and a dose of at least 20mg/kg every 8 hours in children (maximum approved dose is 120 mg/kg daily given in 3 divided doses) are recommended. There is limited safety data available to support a dose of above 2g three times daily (or every 8 hours). Regular sensitivity testing is recommended when treating Pseudomonas aeruginosa infections. **Dosage Schedule for Adults with Impaired Renal Function** Dosage should be reduced in patients with creatinine clearance less than 51 mL/min, as scheduled below. 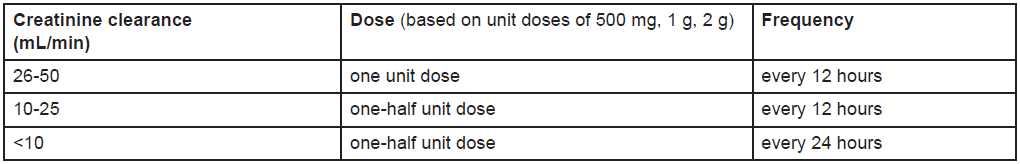 Meropenem is cleared by haemodialysis and haemofiltration; if continued treatment with Meronem IV is necessary, it is recommended that the unit dose (based on the type and severity of infection) is administered at the completion of the haemodialysis procedure to restore therapeutically effective plasma concentrations. There is no experience with the use of Meronem IV in patients under peritoneal dialysis. **Dosage in Adults with Hepatic Insufficiency** No dosage adjustment is necessary in patients with hepatic insufficiency (see “Special warnings and precautions for use” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Elderly Patients** No dosage adjustment is required for the elderly with normal renal function or creatinine clearance values above 50 mL/min. **Children** For children over 3 months and up to 12 years of age the recommended dose is 10–20 mg/kg every 8 hours depending on type and severity of infection, susceptibility of the pathogen and the condition of the patient. In children over 50 kg weight, adult dosage should be used. In meningitis the recommended dose is 40 mg/kg every 8 hours. There is no experience in children with renal impairment. **Method of Administration** Meronem IV can be given as an intravenous bolus injection over approximately 5 minutes or by intravenous infusion over approximately 15 to 30 minutes using the specific available presentations. There is limited safety data available to support the administration of a 40 mg/kg bolus dose. There is limited safety data available to support the administration of a 2g bolus dose. Meronem IV to be used for bolus intravenous injection should be constituted with sterile Water for Injections (5 mL per 250 mg Meropenem). This provides an approximate concentration of 50 mg/mL. Constituted solutions are clear, and colourless or yellow. Meronem IV for intravenous infusion may be constituted with compatible infusion fluids (50 to 200 mL) (see “Incompatibilities and Special precautions for storage” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Meronem IV is indicated for treatment, in adults and children, of the following infections caused by single or multiple bacteria sensitive to meropenem. - Pneumonias and Nosocomial Pneumonias - Urinary Tract Infections - Intra-abdominal Infections - Gynaecological Infections, such as endometritis and pelvic inflammatory disease - Skin and Skin Structure Infections - Meningitis - Septicaemia - Empiric treatment, for presumed infections in adult patients with febrile neutropenia, used as monotherapy or in combination with anti-viral or anti-fungal agents. Meronem IV has proved efficacious alone or in combination with other antimicrobial agents in the treatment of polymicrobial infections. There is no experience in paediatric patients with neutropenia or primary or secondary immunodeficiency.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Hypersensitivity to any other carbapenem antibacterial agent. Severe hypersensitivity (e.g. anaphylactic reaction, severe skin reaction) to any other type of beta-lactam antibacterial agent (e.g. penicillins or cephalosporins).
J01DH02
meropenem
Manufacturer Information
APOTHECA MARKETING PTE LTD
EUGIA PHARMA SPECIALITIES LIMITED
Active Ingredients
Documents
Package Inserts
MEROGRAM POWDER FOR SOLUTION FOR INJECTION PI.pdf
Approved: January 6, 2022
