Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**Posology and method of administration** Therapy should be initiated by a physician experienced in the treatment of patients with haematological malignancies or GIST, as appropriate. The prescribed dose should be administered orally with a meal and a large glass of water to minimize the risk of gastrointestinal disturbances. Doses of 400 mg or 600 mg should be administered once daily, whereas a daily dose of 800 mg should be administered as 400 mg twice a day, in the morning and in the evening. Treatment should be continued as long as the patient continues to benefit. Monitoring of response to imatinib therapy in Ph+ CML patients should be performed routinely and when therapy is modified, to identify suboptimal response, loss of response to therapy, poor patient compliance, or possible drug-drug interaction. Results of monitoring should guide appropriate CML management. **Dosage in CML** The recommended dosage of imatinib is 400 mg/day for adult patients in chronic phase CML and 600 mg/day for patients in accelerated phase or blast crisis. The prescribed dose should be administered orally, once daily with a meal and a large glass of water. Dose increase from 400 mg to 600 mg in patients with chronic phase disease, or from 600 mg to a maximum of 800 mg daily in patients in accelerated phase or blast crisis may be considered in the absence of severe adverse drug reaction and severe non-leukaemia-related neutropenia or thrombocytopenia in the following circumstances: disease progression (at any time); failure to achieve a satisfactory haematological response after at least 3 months of treatment; failure to achieve a cytogenetic response after 12 months of treatment; or loss of a previously achieved haematological and/or cytogenetic response. Dosing in pediatric patients should be on the basis of body surface area (mg/m2). The recommended dose of imatinib for children with newly diagnosed Ph+ CML is 340 mg/m2/day (not to exceed 600mg). Doses of 260 mg/m2/day and 340 mg/m2/day are recommended for children with chronic phase CML and advanced phase CML respectively, after failure of interferon-alpha therapy. However, the total daily dose in children should not exceed adult equivalent doses of 400 mg and 600 mg respectively. Treatment can be given as a once daily dose or alternatively the daily dose may be split into two administrations – one in the morning and one in the evening. The dose recommendation is currently based on a small number of paediatric patients. There is no experience with the treatment of children below 2 years of age. **Dosage in Ph+ ALL** The recommended dose of imatinib is 600 mg/day for adult patients with relapsed/refractory Ph+ ALL. Dosing in pediatric patients should be on the basis of body surface area (mg/m2). The recommended dose of imatinib to be given in combination with chemotherapy to children with newly diagnosed Ph+ALL is 340 mg/m2/day (not to exceed 600mg). Treatment can be given as a once daily dose. The dose recommendation is currently based on a small number of paediatric patients. **Dosage in GIST** The recommended dose of imatinib is 400 mg/day for adult patients with unresectable and/or metastatic, malignant GIST. A dose increase from 400 mg to 600 mg or 800 mg for patients may be considered in the absence of adverse drug reactions if assessments demonstrate an insufficient response to therapy. Treatment with imatinib in GIST patients should be continued until disease progression. The recommended dose of imatinib is 400 mg/day for the adjuvant treatment of adult patients following resection of GIST. Optimal treatment duration is not yet established. Length of treatment in the clinical trial supporting this indication was 36 months. **Dose adjustments for adverse drug reactions** Non-haematological adverse drug reactions If a severe non-hematological adverse drug reaction develops with imatinib use, treatment must be withheld until the event has resolved. Thereafter, treatment can be resumed as appropriate depending on the initial severity of the event. If elevations in bilirubin > 3 x institutional upper limit of normal (IULN) or in liver transaminases > 5 x IULN occur, imatinib should be withheld until bilirubin levels have returned to a < 1.5 x IULN and transaminase levels to < 2.5 x IULN. Treatment with imatinib may then be continued at a reduced daily dose. In adults the dose should be reduced from 400 to 300 mg, or from 600 to 400 mg, or from 800 mg to 600 mg, and in pediatric patients from 260 to 200 mg/m2/day or from 340 to 260 mg/m2/day. Haematological adverse drug reactions Dose reduction or treatment interruption for severe neutropenia and thrombocytopenia are recommended as indicated in the table below. 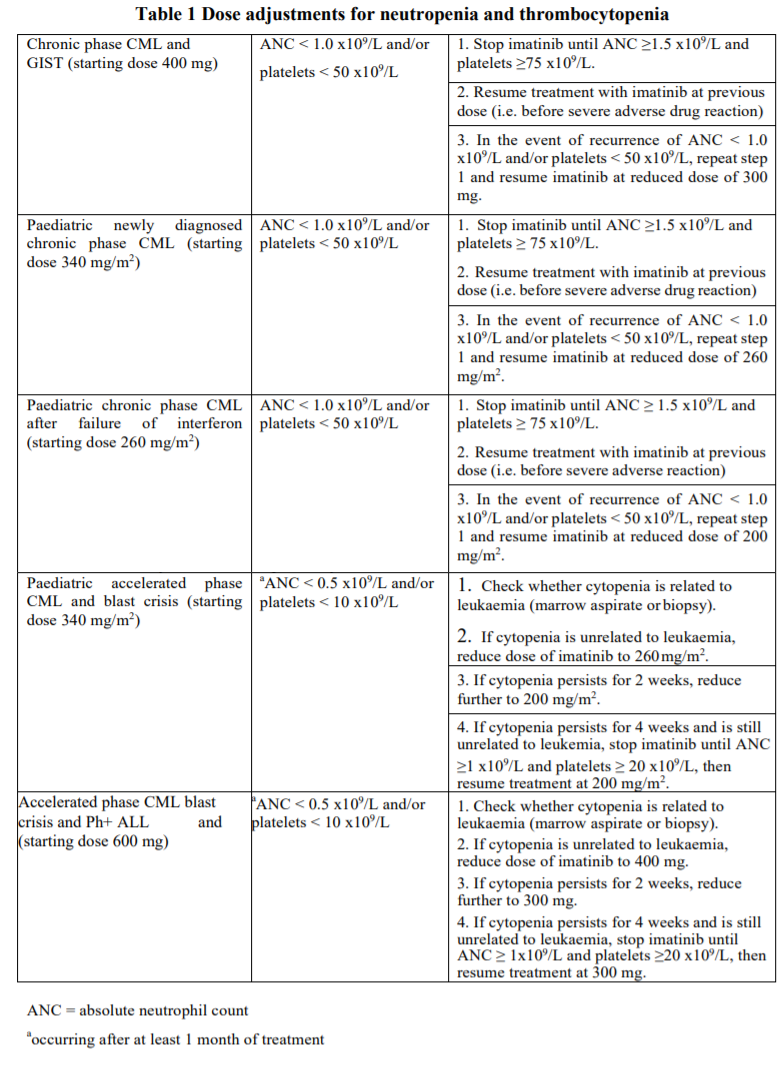 **Special populations** **Pediatric patients (below 18 years)** There is no experience with the use of imatinib in children with CML below 2 years of age and with Ph+ALL below 1 year of age. Dosing in pediatric patients should be on the basis of body surface are (mg/m2). The dose of 340 mg/m2 daily is recommended for pediatric patients with chronic phase and advanced phase CML and Ph+ALL (not to exceed the total dose of 600 mg daily). Treatment can be given as a once daily dose in CML and Ph+ALL. In CML, alternatively the daily dose may be split into two administrations – one in the morning and one in the evening. **Hepatic insufficiency** Imatinib is mainly metabolized by the liver. Patients with mild or moderate liver dysfunction should be given the minimum recommended dose of 400 mg daily, and patients with severe liver dysfunction should start at 300 mg daily. The dose can be reduced if not tolerated. **Renal insufficiency** Imatinib and its metabolites are not significantly excreted via the kidney. Patients with renal dysfunction or on dialysis should be given the minimum recommended dose of 400 mg daily as starting dose. However, in these patients caution is recommended. The dose can be reduced if not tolerated. If tolerated, the dose can be increased for lack of efficacy. **Geriatric patients (65 years or above)** No significant age related pharmacokinetic differences have been observed in adult patients in clinical trials which included over 20% of patients age 65 and older. No specific dose recommendation is necessary in the elderly.
ORAL
Medical Information
**Indications** Imatinib is indicated for the - treatment of adult and paediatric patients with newly diagnosed Philadelphia chromosome positive chronic myeloid leukaemia (Ph+ CML). - treatment of adult and paediatric patients with Ph+ CML in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy. - treatment of paediatric patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) integrated with chemotherapy. - treatment of adult patients with relapsed or refractory Ph+ ALL as monotherapy. - treatment of adult patients with Kit+ (CD117) unresectable and/or metastatic malignant gastrointestinal stromal tumours (GIST). - adjuvant treatment of adult patients following complete gross resection of Kit+ GIST The effectiveness of Imatinib is based on overall haematological and cytogenetic response rates and progression-free survival in CML, on haematological and cytogenetic response rates in relapsed or refractory adult Ph+ ALL, on objective response rates in unresectable and/or metastatic GIST and on recurrence free survival in adjuvant GIST. Except in newly diagnosed chronic phase CML there are no controlled trials demonstrating increased survival.
**CONTRAINDICATIONS** Use in patients with a hypersensitivity to the active substance or to any of the excipients is contraindicated.
L01XE01
xl 01 xe 01
Manufacturer Information
ZYFAS PHARMA PTE. LTD.
Dr Reddy’s Laboratories Ltd
