Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**DOSAGE AND ADMINISTRATION** NEVER ADMINISTER HYPER **RHO** S/D FULL DOSE INTRAVENOUSLY. INJECT ONLY INTRAMUSCULARLY. NEVER ADMINISTER TO THE NEONATE. **Pregnancy and Other Obstetric Conditions** 1. For postpartum prophylaxis, administer one syringe of Hyper **RHO** S/D Full Dose, preferably within 72 hours of delivery. Although a lesser degree of protection is afforded if Rh antibody is administered beyond the 72-hour period, Hyper **RHO** S/D Full Dose may still be given.(7,14) Full-term deliveries can vary in their dosage requirements depending on the magnitude of the fetomaternal hemorrhage. One full dose syringe of Hyper **RHO** S/D Full Dose provides sufficient antibody to prevent Rh sensitization if the volume of red blood cells that has entered the circulation is 15 mL or less.(2–4) In instances where a large (greater than 30 mL of whole blood or 15 mL red blood cells) fetomaternal hemorrhage is suspected, a fetal red cell count by an approved laboratory technique (e.g., modified Kleihauer Betke acid elution stain technique) should be performed to determine the dosage of immune globulin required.(8,15) The red blood cell volume of the calculated fetomaternal hemorrhage is divided by 15 mL to obtain the number of syringes of Hyper **RHO** S/D Full Dose for administration.(3,8,13) If more than 15 mL of red cells is suspected or if the dose calculation results in a fraction, administer the next higher whole number of syringes (e.g., if 1.4, give 2 syringes). 2. For antenatal prophylaxis, one full dose syringe of Hyper **RHO** S/D Full Dose is administered at approximately 28 weeks’ gestation. This must be followed by another full dose, preferably within 72 hours following delivery, if the infant is Rh positive. 3. Following threatened abortion at any stage of gestation with continuation of pregnancy, it is recommended that a full dose of Hyper **RHO** S/D Full Dose be given. If more than 15 mL of red cells is suspected due to fetomaternal hemorrhage, the same dose modification in No. 1 above applies. 4. Following miscarriage, abortion, or termination of ectopic pregnancy at or beyond 13 weeks’ gestation, it is recommended that a Hyper **RHO** S/D Full Dose be given. If more than 15 mL of red cells is suspected due to fetomaternal hemorrhage, the same dose modification in No. 1 above applies. If pregnancy is terminated prior to 13 weeks’ gestation, where licensed, a single dose of Hyper **RHO** ® S/D Mini-Dose may be used instead of Hyper **RHO** S/D Full Dose. 5. Following amniocentesis at either 15 to 18 weeks’ gestation or during the third trimester, or following abdominal trauma in the second or third trimester, it is recommended that a Hyper **RHO** S/D Full Dose be administered. If there is a fetomaternal hemorrhage in excess of 15 mL of red cells, the same dose modification in No. 1 applies. If abdominal trauma, amniocentesis, or other adverse event requires the administration of Hyper **RHO** S/D Full Dose at 13 to 18 weeks’ gestation, another full dose should be given at 26 to 28 weeks. To maintain protection throughout pregnancy, the level of passively acquired anti-Rho(D) should not be allowed to fall below the level required to prevent an immune response to Rh positive red cells. The half-life of IgG is 23 to 26 days. In any case, a Hyper **RHO** S/D Full Dose should be given within 72 hours after delivery if the baby is Rh positive. If delivery occurs within 3 weeks after the last dose, the postpartum dose may be withheld unless there is a fetomaternal hemorrhage in excess of 15 mL of red blood cells.(16) **Transfusion** In the case of a transfusion of Rho(D) positive red cells to an Rho(D) negative recipient, the volume of Rh positive whole blood administered is multiplied by the hematocrit of the donor unit giving the volume of red blood cells transfused. The volume of red blood cells is divided by 15 mL which provides the number of syringes of Hyper **RHO** S/D Full Dose to be administered. If the dose calculated results in a fraction, the next higher whole number of syringes should be administered (e.g., if 1.4, give 2 syringes). Hyper **RHO** S/D Full Dose should be administered within 72 hours after an incompatible transfusion, but preferably as soon as possible. **Injection Procedure** DO NOT INJECT INTRAVENOUSLY. DO NOT INJECT NEONATE. Hyper **RHO** S/D Full Dose is administered **intramuscularly**, preferably in the deltoid muscle of the upper arm or lateral thigh muscle. The gluteal region should not be used as an injection site because of the risk of injury to the sciatic nerve.(17) 1. Single Syringe Dose INJECT ENTIRE CONTENTS OF THE SYRINGE INTO THE INDIVIDUAL INTRAMUSCULARLY. 2. Multiple Syringe Dose 1. Calculate the number of syringes of Hyper **RHO** S/D Full Dose to be given (see Dosage section). 2. The total volume of Hyper **RHO** S/D Full Dose can be given in divided doses at different sites at one time or the total dose may be divided and injected at intervals, provided the total dosage is given within 72 hours of the fetomaternal hemorrhage or transfusion. USING STERILE TECHNIQUE, INJECT THE ENTIRE CONTENTS OF THE CALCULATED NUMBER OF SYRINGES INTRAMUSCULARLY INTO THE PATIENT. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Hyper **RHO** S/D Full Dose is supplied with a syringe and an attached UltraSafe® Needle Guard for your protection and convenience. Please follow instructions below for proper use of syringe and UltraSafe® Needle Guard. **Directions for Syringe Usage** 1. Remove the prefilled syringe from the package. Lift syringe by barrel, **not** by plunger. 2. Twist the plunger rod clockwise until the threads are seated. 3. With the rubber needle shield secured on the syringe tip, push the plunger rod forward a few millimeters to break any friction seal between the rubber stopper and the glass syringe barrel. 4. Remove the needle shield and expel air bubbles. \[Do not remove the rubber needle shield to prepare the product for administration until immediately prior to the anticipated injection time.\] 5. Proceed with hypodermic needle puncture. 6. Aspirate prior to injection to confirm that the needle is not in a vein or artery. 7. Inject the medication. 8. Keeping your hands behind the needle, grasp the guard with free hand and slide forward toward needle until it is completely covered and guard clicks into place. If audible click is not heard, guard may not be completely activated. (See Diagrams A and B) 9. Place entire prefilled glass syringe with guard activated into an approved sharps container for proper disposal. (See Diagram C) 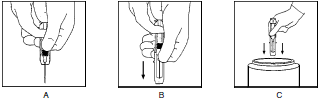 A number of factors could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration, and biological differences in individual patients. Because of these factors, it is important that this product be stored properly and that the directions be followed carefully during use. * * * **REFERENCES** 01. Rho(D) immune globulin (human). _Med Lett Drugs Ther_ 16(1):3–4, 1974. 02. Pollack W, Ascari WQ, Kochesky RJ, et al: Studies on Rh prophylaxis. I. Relationship between doses of anti-Rh and size of antigenic stimulus. _Transfusion_ 11(6):333–9, 1971. 03. Unpublished data on file. 04. The selective use of Rho(D) immune globulin (RhIG). _ACOG Tech Bull_ 61, 1981. 05. Current uses of Rho immune globulin and detection of antibodies. _ACOG Tech Bull_ 35, 1976. 06. Prevention of Rh sensitization. _WHO Tech Rep_ Ser 468:25, 1971. 07. Samson D, Mollison PL: Effect on primary Rh immunization of delayed administration of anti-Rh. _Immunology_ 28:349–57, 1975. 08. Finn R, Harper DT, Stallings SA, et al: Transplacental hemorrhage. _Transfusion_ 3(2):114–24, 1963. 09. Garraty G (ed.): Hemolytic disease of the newborn. Arlington, VA, American Association of Blood Banks, 1984, p 78. 10. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP): General recommendations on immunization. _MMWR_ 2002: 51(RR02), 1–36.
INTRAMUSCULAR
Medical Information
**INDICATIONS AND USAGE** **Pregnancy and Other Obstetric Conditions** Hyper **RHO** S/D Full Dose is recommended for the prevention of Rh hemolytic disease of the newborn by its administration to the Rho(D) negative mother within 72 hours after birth of an Rho(D) positive infant,(12) providing the following criteria are met: 1. The mother must be Rho(D) negative and must not already be sensitized to the Rho(D) factor. 2. Her child must be Rho(D) positive, and should have a negative direct antiglobulin test (see PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If Hyper **RHO** S/D Full Dose is administered antepartum, it is essential that the mother receive another dose of Hyper **RHO** S/D Full Dose after delivery of an Rho(D) positive infant. If the father can be determined to be Rho(D) negative, Hyper **RHO** S/D Full Dose need not be given. Hyper **RHO** S/D Full Dose should be administered within 72 hours to all nonimmunized Rho(D) negative women who have undergone spontaneous or induced abortion, following ruptured tubal pregnancy, amniocentesis or abdominal trauma unless the blood group of the fetus or the father is known to be Rho(D) negative.(7,8) If the fetal blood group cannot be determined, one must assume that it is Rho(D) positive,(2) and Hyper **RHO** S/D Full Dose should be administered to the mother. **Transfusion** Hyper **RHO** S/D Full Dose may be used to prevent isoimmunization in Rho(D) negative individuals who have been transfused with Rho(D) positive red blood cells or blood components containing red blood cells.(5,13) * * * **REFERENCES** 1. Rho(D) immune globulin (human). _Med Lett Drugs Ther_ 16(1):3–4, 1974. 2. Pollack W, Ascari WQ, Crispen JF, et al: Studies on Rh prophylaxis. II. Rh immune prophylaxis after transfusion with Rh-positive blood. _Transfusion_ 11(6):340–4, 1971. 3. The selective use of Rho(D) immune globulin (RhIG). _ACOG Tech Bull_ 61, 1981. 4. Current uses of Rho immune globulin and detection of antibodies. _ACOG Tech Bull_ 35, 1976. 5. Ascari WQ, Allen AE, Baker WJ, et al: Rho(D) immune globulin (human): evaluation in women at risk of Rh immunization. _JAMA_ 205(1):1–4, 1968. 6. Prevention of Rh sensitization. _WHO Tech Rep Ser_ 468:25, 1971.
**CONTRAINDICATIONS** None known.
J06BB01
anti-D (rh) immunoglobulin
Manufacturer Information
GRIFOLS ASIA PACIFIC PTE. LTD.
Grifols Therapeutics LLC
Active Ingredients
Documents
Package Inserts
HyperRHO SD Injection PI.pdf
Approved: June 10, 2019
