Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**4.2 Posology and method of administration** Dosing guidelines - Dosing should be individualized and titrated to desired clinical response. - This medicinal product is not indicated for infusions lasting longer than 24 hours. - This medicinal product should be administered using a controlled infusion device. Posology 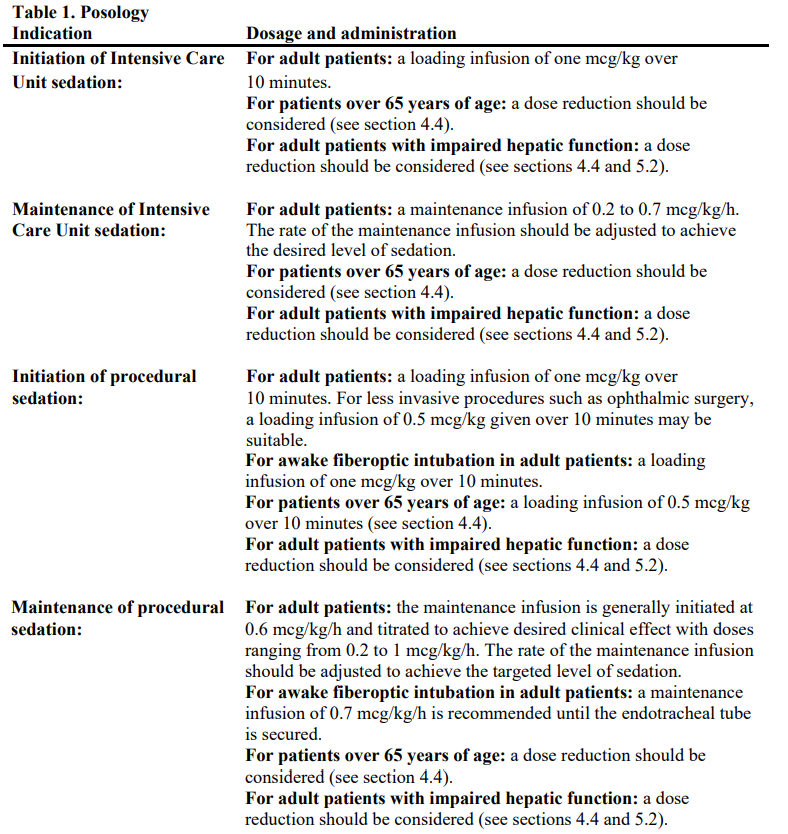 Dosage adjustment Due to possible pharmacodynamic interactions, a reduction in dosage of dexmedetomidine or other concomitant anaesthetics, sedatives, hypnotics or opioids may be required when co-administered (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dosage reductions may need to be considered for adult patients with hepatic impairment, and geriatric patients (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration This medicine must be administered only as a diluted intravenous infusion using a controlled infusion device. For instructions on dilution of the medicinal product before administration, see section 6.7 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** _Intensive Care Unit sedation_ Dexmedetomidine is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting. This medicinal product should be administered by continuous infusion not to exceed 24 hours. Dexmedetomidine has been continuously infused in mechanically ventilated patients prior to extubation, during extubation, and post-extubation. It is not necessary to discontinue dexmedetomidine prior to extubation. _Procedural sedation_ Dexmedetomidine is indicated for sedation of non-intubated adult patients prior to and/or during surgical and other procedures.
**4.3 Contraindications** None
N05CM18
dexmedetomidine
Manufacturer Information
GOLDPLUS UNIVERSAL PTE LTD
HBM Pharma s.r.o.
Active Ingredients
Documents
Package Inserts
Dexmedetomidine PI.pdf
Approved: April 21, 2023
