Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INHALANT
**DOSAGE AND ADMINISTRATION** **FOR USE ONLY AS AN ANALGESIC AGENT (SEE CONTRAINDICATION)** **For Adults and Adolescents aged 12 Years and Above** _**Dosage:**_ One bottle of PENTHROX (3 ml) to be vaporised in a PENTHROX Inhaler. On finishing the initial bottle (3 ml), another bottle (3 ml) may be used. Up to 6 ml may be administered per day. The refilling must be conducted in a well-ventilated area to reduce environmental exposure to methoxyflurane vapour. To maximise safety, the lowest effective dosage of PENTHROX (methoxyflurane) to provide analgesia should be used, particularly for adolescents and the elderly. The total weekly dose should not exceed 15 ml. Administration of consecutive days is not recommended. The cumulative dose received by patients receiving intermittent doses of PENTHROX (methoxyflurane) for painful procedures must be carefully monitored to ensure that the recommended dose of methoxyflurane is not exceeded. Methoxyflurane may cause renal failure if the recommended dose is exceeded. Methoxyflurane-associated renal failure is generally irreversible. **_Administration:_** PENTHROX (methoxyflurane) is self-administered under observation (and assisted if necessary) by a person trained in its administration using the handheld PENTHROX Inhaler. Instructions on the preparation of the PENTHROX Inhaler and correct administration are provided in Figure 1. 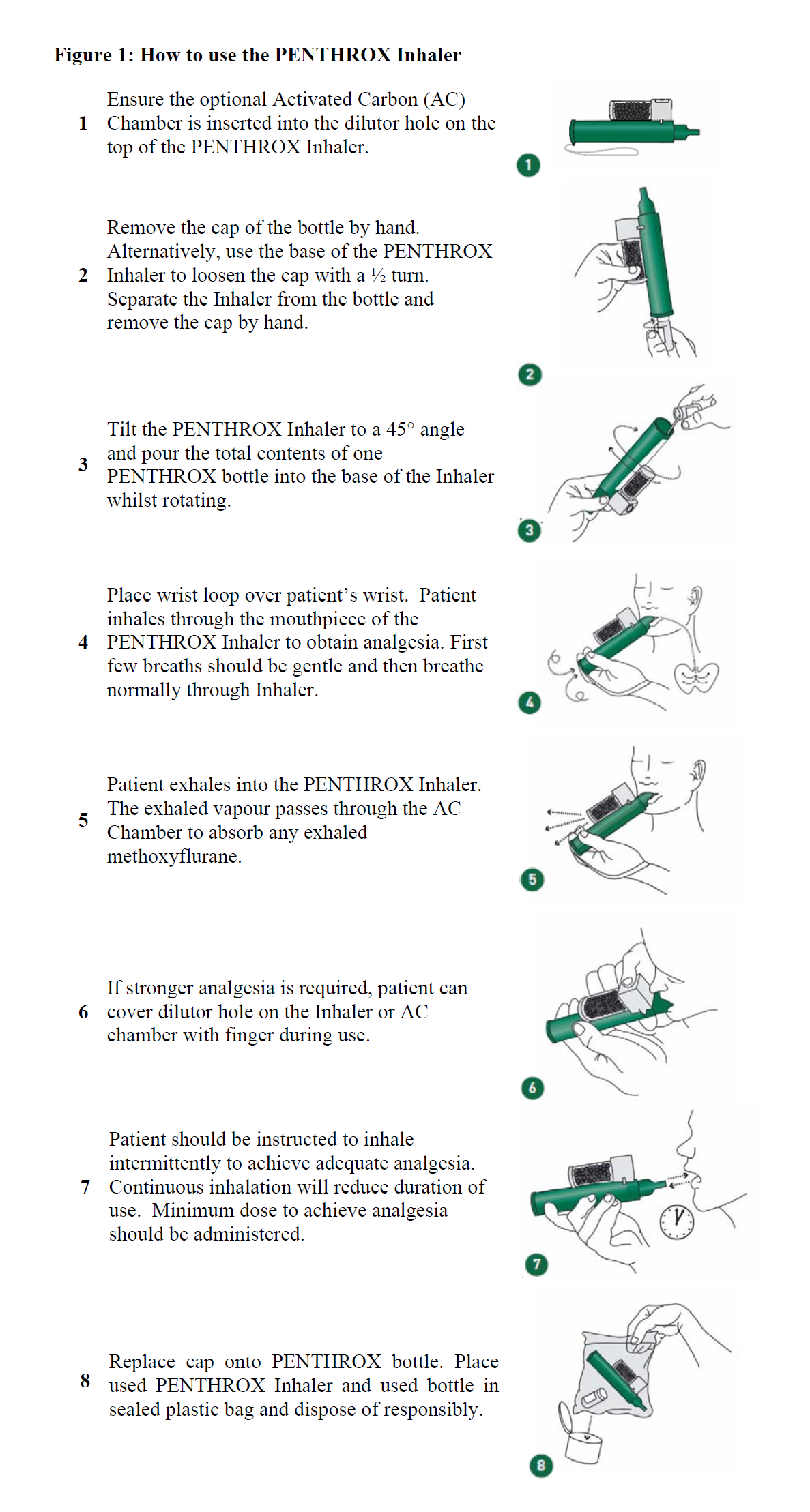 Pain relief will commence after approximately 6–10 inhalations. PENTHROX can be inhaled continuously or intermittently. For intermittent administration, a top-up of six inhalations may be taken before each of the more painful parts of the procedure.
RESPIRATORY (INHALATION)
Medical Information
**INDICATIONS** 1. For emergency relief of moderate pain by self-administration in conscious haemodynamically stable patients with trauma and associated pain, under supervision of personnel trained in its use (see Dosage and Administration) 2. For the relief of moderate pain in monitored conscious patients who require analgesia for surgical procedures (See Dosage and Administration) **Note:** the total maximum dose must not be exceeded.
**CONTRAINDICATIONS** - Use as an anaesthetic agent - Clinically significant renal impairment - Hypersensitivity to fluorinated anaesthetics or any ingredients in PENTHROX - Clinically evident cardiovascular instability - Clinically evident respiratory depression - Altered level of consciousness due to any cause including head injury, drugs or alcohol - Malignant hyperthermia: patients with known or genetically susceptible to malignant hyperthermia
N02BG09
methoxyflurane
Manufacturer Information
LINK HEALTHCARE SINGAPORE PTE. LTD.
Medical Developments International Limited (MDI)
Active Ingredients
Documents
Package Inserts
Penthrox_SG_PI_v8-clean_July2021.pdf
Approved: August 3, 2021
