Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Posology and method of administration** Eltrombopag treatment should be initiated and remain under the supervision of a physician who is experienced in the treatment of haematological diseases or management of chronic hepatitis C and its complications. Eltrombopag dosing requirements must be individualised based on the patient's platelet counts. The objective of treatment with Eltrombopag should not be to normalise platelet counts. Eltrombopag should be taken at least two hours before or four hours after any products such as antacids, dairy products, or mineral supplements containing polyvalent cations (e.g. aluminum, calcium, iron, magnesium, selenium and zinc) ( _see Interactions, Pharmacokinetics – Absorption_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Eltrombopag may be taken with food containing little (< 50 mg) or preferably no calcium ( _see Interactions, Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Immune thrombocytopenia** Use the lowest dose of Eltrombopag to achieve and maintain a platelet count ≥50,000/microlitre. Dose adjustments are based upon the platelet count response. Do not use Eltrombopag to normalize platelet counts. In clinical studies, platelet counts generally increased within 1 to 2 weeks after starting Eltrombopag and decreased within 1 to 2 weeks after discontinuation. _Initial Dose Regimen_ The recommended starting dose of Eltrombopag is 50 mg once daily. For patients of East-/Southeast-Asian ancestry, Eltrombopag should be initiated at a reduced dose of 25 mg once daily ( _see Pharmacokinetic properties, Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Monitoring and dose adjustment_ After initiating Eltrombopag, adjust the dose to achieve and maintain a platelet count ≥50,000/microlitre as necessary to reduce the risk for bleeding. Do not exceed a dose of 75 mg daily. Clinical haematology and liver function tests should be monitored regularly throughout therapy with Eltrombopag and the dose regimen of Eltrombopag modified based on platelet counts as outlined in Table 1. During therapy with Eltrombopag, complete blood counts (CBCs), including platelet count and peripheral blood smears, should be assessed weekly until a stable platelet count (≥50,000/microlitre for at least 4 weeks) has been achieved. CBCs including platelet counts and peripheral blood smears should be obtained monthly thereafter. 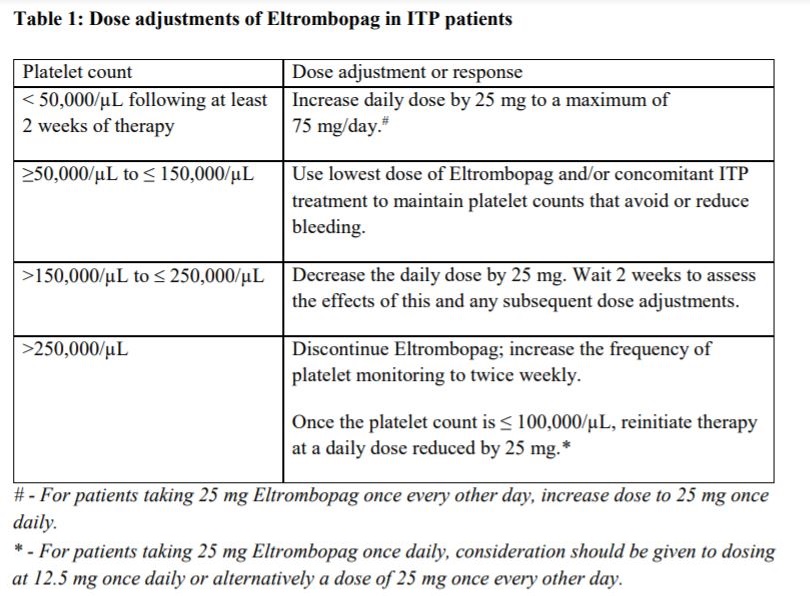 Eltrombopag can be administered in addition to other ITP medicinal products. Modify the dose regimen of concomitant ITP medicinal products, as medically appropriate, to avoid excessive increases in platelet counts during therapy with Eltrombopag. It is necessary to wait for at least 2 weeks to see the effect of any dose adjustment on the patient's platelet response prior to considering another dose increase. The standard Eltrombopag dose adjustment, either decrease or increase, would be 25 mg once daily. _Discontinuation_ Treatment with Eltrombopag should be discontinued if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after four weeks of Eltrombopag therapy at 75 mg once daily. Patients should be clinically evaluated periodically and continuation of treatment should be decided on an individual basis by the treating physician. In non-splenectomised patients, this should include evaluation relative to splenectomy. The reoccurrence of thrombocytopenia is possible upon discontinuation of treatment ( _see Special warnings & precautions for use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Chronic hepatitis C (HCV) associated thrombocytopenia When Eltrombopag is given in combination with antiviral therapies, reference should be made to the full prescribing information of the respective coadministered medicinal products for comprehensive details of administration. Use the lowest dose of Eltrombopag to achieve and maintain a platelet count necessary to initiate and optimise antiviral therapy. Dose adjustments are based upon the platelet count response. Do not use Eltrombopag to normalize platelet counts. In clinical studies, platelet counts generally increased within 1 week of starting Eltrombopag. _**Adult**_ _Initial Dose Regimen_ Initiate Eltrombopag at a dose of 25 mg once daily. No dosage adjustment is necessary for HCV patients of East-/Southeast-Asian ancestry, or patients with mild hepatic impairment. _Monitoring and dose adjustment_ Adjust the dose of Eltrombopag in 25 mg increments every 2 weeks as necessary to achieve the target platelet count required to initiate antiviral therapy (see Table 2). Monitor platelet counts every week prior to starting antiviral therapy. During antiviral therapy adjust the dose of Eltrombopag as necessary to avoid dose reduction of peginterferon. Monitor platelet counts weekly during antiviral therapy until a stable platelet count is achieved. CBC's, including platelet counts and peripheral blood smears, should be obtained monthly thereafter. Do not exceed a dose of 100 mg Eltrombopag once daily. For specific dosage instructions for peginterferon alfa or ribavirin, refer to their respective prescribing information. 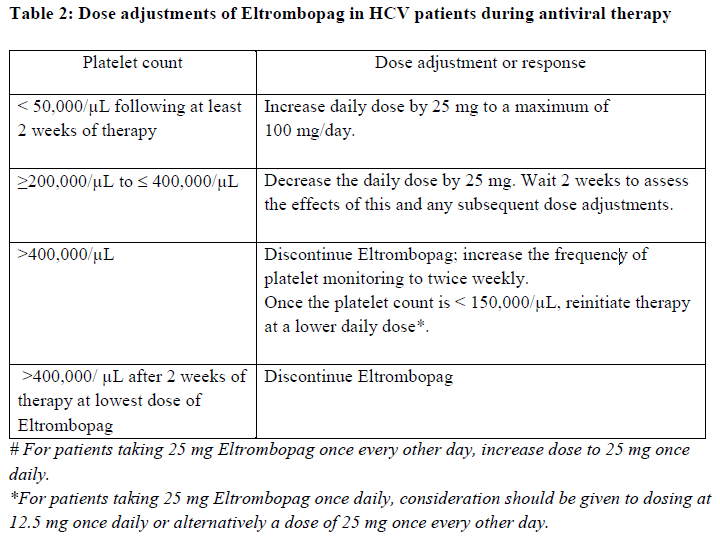 _Discontinuation_ In patients with HCV genotype 1/4/6, independent of the decision to continue interferon therapy, discontinuation of Eltrombopag therapy should be considered in patients who do not achieve virological response at week 12. If HCV-RNA remains detectable after 24 weeks of treatment, Eltrombopag therapy should be discontinued. Eltrombopag treatment should be terminated when antiviral therapy is discontinued. Excessive platelet count responses, as outlined in Table 2, or important liver test abnormalities may also necessitate discontinuation of Eltrombopag ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Children**_ The safety and efficacy of Eltrombopag in children with chronic HCV have not been established. **First-line Severe Aplastic Anemia** Initiate Eltrombopag concurrently with standard immunosuppressive therapy. _Initial Dose Regimen:_ The recommended initial dose regimen is listed in Table 3. Do not exceed the initial dose of Eltrombopag.  For patients with severe aplastic anemia of East-/Southeast-Asian ancestry or those with mild, moderate or severe hepatic impairment (Child-Pugh Class A, B, C), decrease the initial Eltrombopag dose by 50 % as listed in Table 4. If baseline ALT or AST levels are > 6 x ULN, do not initiate Eltrombopag until transaminase levels are < 5 x ULN. Determine the initial dose for these patients based on Table 3 or Table 4.  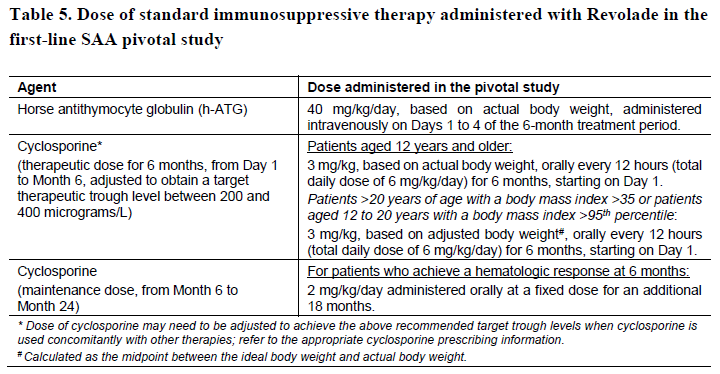 _Monitoring and Dose Adjustment:_ Perform clinical hematology and liver tests regularly throughout therapy with Eltrombopag. 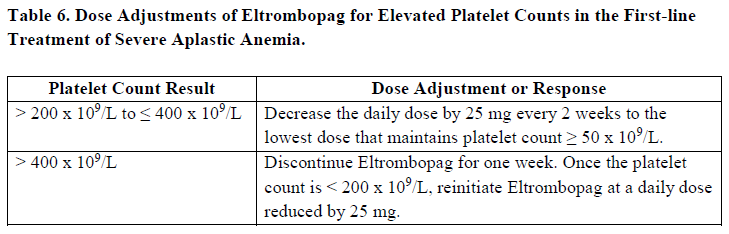 Table 7 summarises the recommendations for dose interruption, reduction, or discontinuation of Eltrombopag in the management of elevated liver transaminase levels and thromboembolic events. 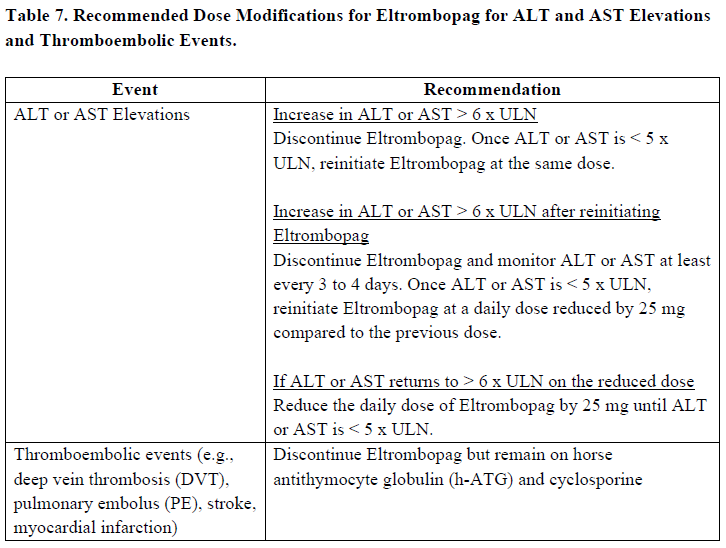 The total duration of Eltrombopag treatment is 6 months. **Refractory Severe Aplastic Anaemia** _**Adults**_ _Initial Dose Regimen_ Initiate Eltrombopag at a dose of 50 mg once daily. For SAA patients of East-/Southeast-Asian ancestry, Eltrombopag should be initiated at a dose of 25 mg once daily ( _see Pharmacokinetic, Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Monitoring and dose adjustment_ Haematological response requires dose titration, generally up to 150 mg, and may take up to 16 weeks after starting Eltrombopag ( _see Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Adjust the dose of Eltrombopag in 50 mg increments every 2 weeks as necessary to achieve the target platelet count ≥ 50,000/microlitre. Do not exceed a dose of 150 mg daily. Monitor clinical haematology and liver tests regularly throughout therapy with Eltrombopag and modify the dosage regimen of Eltrombopag based on platelet counts as outlined in Table 8. 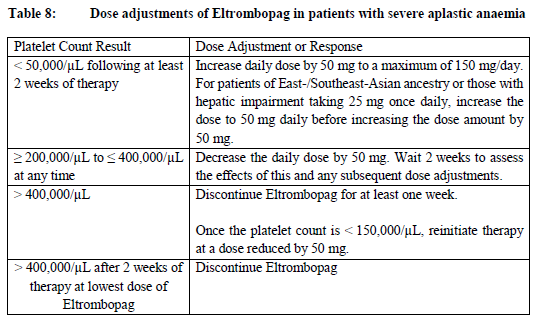 _Tapering for tri-lineage (white blood cells, red blood cells, and platelets) responders_ Once platelet count > 50,000/microlitre, haemoglobin > 10 g/dL in the absence of red blood cell (RBC) transfusion, and absolute neutrophil (ANC) > 1 x 109/L for more than 8 weeks, the dose of Eltrombopag should be reduced by up to 50%. If counts stay stable after 8 weeks at the reduced dose, then discontinue Eltrombopag, and monitor blood counts. If platelet counts drop to < 30,000/microlitre, haemoglobin to < 9 g/dL or ANC < 0.5 x 109/L, Eltrombopag may be reinitiated at the previous dose. _Discontinuation_ If no haematological response has occurred after 16 weeks of therapy with Eltrombopag, discontinue therapy. Consider Eltrombopag discontinuation if new cytogenetic abnormalities are observed ( _see Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Excessive platelet count responses (as outlined in Table 3) or important liver test abnormalities also necessitate discontinuation of Eltrombopag ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Children**_ The safety and efficacy of Eltrombopag in children with SAA have not been established. **_Other_ Populations _(All Indications)_** _Renal impairment_ No dose adjustment is necessary in patients with renal impairment. Patients with impaired renal function should use Eltrombopag with caution and close monitoring, for example by testing serum creatinine and/or performing urine analysis ( _see Pharmacokinetic, Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ ITP patients with liver cirrhosis (hepatic impairment, Child-Pugh score ≥5) should use Eltrombopag with caution and close monitoring ( _see Warnings and Precautions, Pharmacokinetics, Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the use of Eltrombopag is deemed necessary for ITP patients with hepatic impairment the starting dose must be 25 mg once daily. After initiating the dose of Eltrombopag in patients with hepatic impairment wait 3 weeks before increasing the dose. Thrombocytopenic patients with chronic HCV with hepatic impairment should initiate Eltrombopag at a dose of 25 mg once daily ( _see Pharmacokinetics, Special Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The risk of thromboembolic events (TEEs) has been found to be increased in thrombocytopenic patients (platelet count < 50,000/microlitre) with chronic liver disease (CLD), without concomitant ITP, treated with 75 mg Eltrombopag once daily for two weeks in preparation for invasive procedures ( _see Special warnings & precautions for use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Chronic HCV patients with hepatic impairment and refractory severe aplastic anaemia patients with hepatic impairment should initiate Eltrombopag at a dose of 25 mg once daily ( _see Pharmacokinetic, Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In a clinical trial in definitive immunosuppressive therapy-naïve severe aplastic anemia patients with baseline AST/ALT > 5 x ULN were ineligible to participate. The initial dose of Eltrombopag in patients with hepatic impairment in the first-line setting should be determined as necessary based on clinical judgement, tolerability, and close monitoring of liver function. _Paediatric population_ The safety and efficacy of Eltrombopag have not been established in paediatric ITP patients younger than one year. In paediatric clinical studies, subjects between 1 to 5 years of age were administered Eltrombopag as a _powder for oral suspension_ formulation. Eltrombopag is only available as tablets and cannot be used in patients who are unable to swallow Eltrombopag tablets whole. The safety and efficacy of Eltrombopag in paediatric patients with chronic HCV related thrombocytopenia or SAA have not been established. _Elderly_ There are limited data on the use of Eltrombopag in subjects aged 65 years and older. In the clinical studies of Eltrombopag, overall, no clinically significant differences in safety of Eltrombopag were observed between subjects aged at least 65 years and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out ( _see Pharmacokinetics, Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _East-/Southeast-Asian patients_ For adult and pediatric patients of East-/Southeast-Asian ancestry, Revolade should be initiated at a dose of 25mg once daily for the treatment of ITP, HCV-associated thrombocytopenia, and refractory SAA. For the treatment of patients with first-line SAA refer to _Posology and method of administration_.
ORAL
Medical Information
**Therapeutic Indications** Revolade is indicated for immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments (e.g. corticosteroids, immunoglobulins). Revolade is indicated for immune thrombocytopenia (ITP) lasting 6 months or longer from diagnosis in paediatric patients aged 6 years and above who are refractory to other treatments (e.g. corticosteroids, immunoglobulins). Revolade is indicated in adult patients with chronic hepatitis C virus (HCV) infection for the treatment of thrombocytopenia, where the degree of thrombocytopenia is the main factor preventing the initiation or limiting the ability to maintain optimal interferon-based therapy. Revolade is indicated in combination with standard immunosuppressive therapy for the first-line treatment of adult and adolescent patients 12 years and older with severe aplastic anemia. Revolade is indicated in adult patients with acquired severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for haematopoietic stem cell transplantation.
**Contraindications** Hypersensitivity to Eltrombopag or to any of the excipients.
B02BX05
eltrombopag
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Glaxo Operations UK Limited (trading as Glaxo Wellcome Operations)
Glaxo Wellcome S.A. (primary & secondary packager)
Siegfried Barbera S.L.
Lek d.d
Active Ingredients
Documents
Package Inserts
Revolade PI.pdf
Approved: November 30, 2020
