Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** **Usual Dosage for Adults** Smoking cessation therapies are more likely to succeed for patients who are motivated to stop smoking and who are provided additional advice and support. Patients should be provided with appropriate educational materials and counseling to support the quit attempt. The patient should set a date to stop smoking. CHAMPIX dosing should start one week before this date. Alternatively, the patient can begin CHAMPIX dosing and then quit smoking between days 8 and 35 of treatment (see section **5.1 Pharmacodynamic properties** – _Flexibility in Setting a Quit Date_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended dose of CHAMPIX is 1 mg twice daily following a 1-week titration as follows: 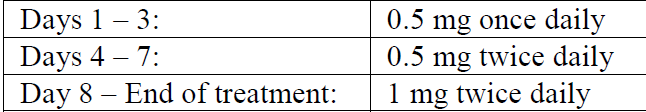 Patients should be treated with CHAMPIX for 12 weeks. For patients who have successfully stopped smoking at the end of 12 weeks, an additional course of 12 weeks treatment with CHAMPIX at 1 mg twice daily is recommended for the maintenance of abstinence (see section **5.1 Pharmacodynamic properties** – _Maintenance of Abstinence Study_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). A gradual approach to quitting smoking with CHAMPIX should be considered for patients who are not able or willing to quit abruptly. Patients should reduce smoking during the first 12 weeks of treatment and quit by the end of that treatment period. Patients should then continue taking CHAMPIX for an additional 12 weeks for a total of 24 weeks of treatment (see section **5.1 Pharmacodynamic properties** – _Gradual approach to quitting smoking_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients who do not succeed in stopping smoking during 12 weeks of initial therapy, or who relapse after treatment, should be encouraged to make another attempt once factors contributing to the failed attempt have been identified and addressed (see section **5.1 Pharmacodynamic properties** – _Study in Subjects Re-treated with CHAMPIX_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients who cannot tolerate adverse effects of CHAMPIX may have the dose lowered temporarily or permanently. CHAMPIX tablets should be swallowed whole with water. CHAMPIX can be taken with or without food. _Patients with renal insufficiency:_ No dosage adjustment is necessary for patients with mild to moderate renal impairment. For patients with severe renal impairment, dosing should begin at 0.5 mg once daily for the first 3 days then increased to 1 mg once daily. There is insufficient clinical experience with varenicline in patients with end stage renal disease (see section **5.2 Pharmacokinetic properties** – _Patients with renal insufficiency_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients with hepatic impairment:_ No dosage adjustment is necessary for patients with hepatic impairment (see section **5.2 Pharmacokinetic properties** – _Patients with hepatic impairment_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Use in elderly patients:_ No dosage adjustment is necessary for elderly patients. Because elderly patients are more likely to have decreased renal function, prescribers should consider the renal status of an elderly patient (see above Patients with renal insufficiency and section **5.2 Pharmacokinetic properties** – _Patients with renal insufficiency and Use in elderly patients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Use in pediatric patients:_ Safety and effectiveness of CHAMPIX in pediatric patients have not been established; therefore, CHAMPIX is not recommended for use in patients under 18 years of age.
ORAL
Medical Information
**4.1 Therapeutic indications** CHAMPIX is indicated as an aid to smoking cessation treatment.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients.
N07BA03
varenicline
Manufacturer Information
PFIZER PRIVATE LIMITED
Pfizer Italia S.r.l.
