Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Treatment with GONAL-f™ should be initiated under the supervision of a physician experienced in the treatment of fertility disorders. Posology The dose recommendations given for GONAL-f™ are those in use for urinary FSH. Clinical assessment of GONAL-f™ indicates that its daily doses, regimens of administration, and treatment monitoring procedures should not be different from those currently used for urinary FSH-containing medicinal products. It is advised to adhere to the recommended starting doses indicated below. Comparative clinical studies have shown that on average patients require a lower cumulative dose and shorter treatment duration with GONAL-f™ compared with urinary FSH. Therefore, it is considered appropriate to give a lower total dose of GONAL-f™ than generally used for urinary FSH, not only in order to optimise follicular development but also to minimise the risk of unwanted ovarian hyperstimulation. See section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Bioequivalence has been demonstrated between equivalent doses of the monodose presentation and the multidose presentation of GONAL-f™. The following table states the volume to be administered to deliver the prescribed dose: 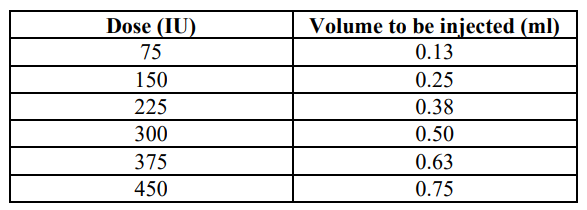 The next injection should be done at the same time the next day. Women with anovulation (including polycystic ovarian syndrome) GONAL-f™ may be given as a course of daily injections. In menstruating women treatment should commence within the first 7 days of the menstrual cycle. A commonly used regimen commences at 75–150 international units FSH daily and is increased preferably by 37.5 or 75 international units at 7 or preferably 14 day intervals if necessary, to obtain an adequate, but not excessive, response. Treatment should be tailored to the individual patient’s response as assessed by measuring follicle size by ultrasound and/or oestrogen secretion. The maximal daily dose is usually not higher than 225 international units FSH. If a patient fails to respond adequately after 4 weeks of treatment, that cycle should be abandoned and the patient should undergo further evaluation after which she may recommence treatment at a higher starting dose than in the abandoned cycle. When an optimal response is obtained, a single injection of 250 micrograms recombinant human choriogonadotropin alfa (r-hCG) or 5,000 international units, up to 10,000 international units hCG should be administered 24–48 hours after the last GONAL-f™ injection. The patient is recommended to have coitus on the day of, and the day following, hCG administration. Alternatively intrauterine insemination may be performed. If an excessive response is obtained, treatment should be stopped and hCG withheld (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment should recommence in the next cycle at a dose lower than that of the previous cycle. Women undergoing ovarian stimulation for multiple follicular development prior to _in vitro_ fertilisation or other assisted reproductive technologies: A commonly used regimen for superovulation involves the administration of 150–225 international units of GONAL-f™ daily, commencing on days 2 or 3 of the cycle. Treatment is continued until adequate follicular development has been achieved (as assessed by monitoring of serum oestrogen concentrations and/or ultrasound examination), with the dose adjusted according to the patient’s response, to usually not higher than 450 international units daily. In general adequate follicular development is achieved on average by the tenth day of treatment (range 5 to 20 days). A single injection of of 250 micrograms r-hCG or 5,000 international units up to 10, 000 international units hCG is administered 24–48 hours after the last GONAL-f™ injection to induce final follicular maturation. Down-regulation with a gonadotropin-releasing hormone (GnRH) agonist or antagonist is now commonly used in order to suppress the endogenous LH surge and to control tonic levels of LH. In a commonly used protocol, GONAL-f™ is started approximately 2 weeks after the start of agonist treatment, both being continued until adequate follicular development is achieved. For example, following two weeks of treatment with an agonist, 150–225 international units GONAL-f™ are administered for the first 7 days. The dose is then adjusted according to the ovarian response. Overall experience with IVF indicates that in general the treatment success rate remains stable during the first four attempts and gradually declines thereafter. Special population _Elderly population_ There is no relevant use of GONAL-f™ in the elderly population. Safety and effectiveness of GONAL-f™ in elderly patients have not been established. _Renal or hepatic impairment_ Safety, efficacy and pharmacokinetics of GONAL-f™ in patients with renal or hepatic impairment have not been established. Paediatric population There is no relevant use of GONAL-f™ in the paediatric population. Method of administration GONAL-f™ is intended for subcutaneous administration. The first injection of GONAL-f™ should be performed under direct medical supervision. Self-administration of GONAL-f™ should only be performed by patients who are well motivated, adequately trained and have access to expert advice. As GONAL-f multidose is intended for several injections, clear instructions should be provided to the patients to avoid misuse of the multidose presentation. Due to a local reactivity to benzyl alcohol, the same site of injection should not be used on consecutive days. Individual reconstituted vials should be for single patient use only. For instructions on the reconstitution and administration of GONAL-f™ powder and solvent for solution for injection see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** In adult women - Anovulation (including polycystic ovarian syndrome) in women who have been unresponsive to treatment with clomiphene citrate. - Stimulation of multifollicular development in women undergoing superovulation for assisted reproductive technologies (ART) such as _in vitro_ fertilisation (IVF), gamete intra-fallopian transfer and zygote intra-fallopian transfer.
**4.3 Contraindications** GONAL-f™ must not be used in: - hypersensitivity to the active substance follitropin alfa, FSH or to any of the excipients - tumours of the hypothalamus or pituitary gland In women: - ovarian enlargement or ovarian cyst not due to polycystic ovarian disease - gynaecological haemorrhages of unknown aetiology - ovarian, uterine or mammary carcinoma GONAL-f™ should not be used when an effective response cannot be obtained, such as: In women: - primary ovarian failure - malformations of sexual organs incompatible with pregnancy - fibroid tumours of the uterus incompatible with pregnancy
G03GA05
follitropin alfa
Manufacturer Information
MERCK PTE. LTD.
Merck Serono S.p.A.
Active Ingredients
Documents
Package Inserts
Gonal-F Injection 1050iu PI.pdf
Approved: October 6, 2017
