Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**4.2 Posology and method of administration** Oral dosage forms of methotrexate should not be split or crushed but should be taken whole. **Anti-neoplastic chemotherapy** Oral administration in tablet form is often preferred since absorption is rapid and effective serum levels are obtained. _**Choriocarcinoma and similar trophoblastic diseases:**_ Methotrexate is administered orally in doses of 15 to 30 mg daily for a 5-day course. Such courses are usually repeated for 3 to 5 times as required, with rest periods of one or more weeks interposed between courses, until any manifesting toxic symptoms subside. The effectiveness of therapy is ordinarily evaluated by 24-hour quantitative analysis of urinary chorionic gonadotropin hormone (CGH), which should return to normal or less than 50 international units/24 h, usually after the 3rd or 4th course and usually be followed by a complete resolution of measurable lesions in 4 to 6 weeks. One to two courses of methotrexate after normalization of CGH is usually recommended. Before each course of the drug careful clinical assessment is essential. Cyclic combination therapy of methotrexate with other antitumor drugs has been reported as being useful. Since hydatidiform mole may precede or be followed by choriocarcinoma, prophylactic chemotherapy with methotrexate has been recommended. Chorioadenoma destruens is considered to be an invasive form of hydatidiform mole. Methotrexate is administered in these disease states in doses similar to those recommended for choriocarcinoma. **_Leukemia:_** Acute lymphatic (lymphoblastic) leukemia in children and young adolescents is the most responsive to present day chemotherapy. In young adults and older patients, clinical remission is more difficult to obtain and early relapse is more common. In chronic lymphatic leukemia, the prognosis for adequate response is less encouraging. Methotrexate alone or in combination with steroids was used initially for induction of remission of lymphoblastic leukemias. More recently corticosteroid therapy in combination with other antileukemic drugs or in cyclic combinations with methotrexate included appear to produce rapid and effective remissions. When used for induction, methotrexate alone or in combination with other agents appears to be the drug of choice for securing maintenance of drug induced remissions. When remission is achieved and supportive care has produced general clinical improvement, maintenance therapy is initiated, as follows: methotrexate is administered 2 times weekly either by mouth. If and when relapse does occur, reinduction of remission can again usually be obtained by repeating the initial induction regimen. Various experts have recently introduced a variety of dosing schedules for both induction and maintenance of remission with various combinations of alkylating and antifolic agents. Multiple drug therapy with several agents, including methotrexate given concomitantly is gaining increasing support in both the acute and chronic forms of leukemia. The physician should familiarize himself with the new advances in antileukemic therapy. Acute granulocytic leukemia is rare in children but common in adults. This form of leukemia responds poorly to chemotherapy and remissions are short with relapses common, and resistance to therapy develops rapidly. **_Meningeal leukemia:_** Patients with leukemia are subject to leukemic invasion of the central nervous system. This may manifest characteristic signs or symptoms or may remain silent and be diagnosed only by examination of the cerebrospinal fluid (CSF) which contains leukemic cells in such cases. Therefore, the CSF should be examined in all leukemic patients. For the treatment of meningeal leukemia, methotrexate is given at intervals of 2 to 5 days. Methotrexate is administered until the cell count of the CSF returns to normal. At this point one additional dose is advisable. For prophylaxis against meningeal leukemia, the dosing is the same as for treatment except for the intervals of administration. On this subject, it is advisable for the physician to consult the medical literature. _**Lymphomas:**_ In Burkitt’s Tumor, Stages I-II, methotrexate has produced prolonged remissions in some cases. Recommended dosing is 10 to 25 mg per day orally for 4 to 8 days. In Stage III, methotrexate is commonly given concomitantly with other antitumor agents. Treatment in all stages usually consists of several courses of the drug interposed with 7 to 10 days rest periods. Lymphosarcomas in Stage III may respond to combined drug therapy with methotrexate given in doses of 0.625 mg to 2.5 mg/kg daily. Hodgkin’s Disease responds poorly to methotrexate and to most types of chemotherapy. _**Mycosis fungoides:**_ Therapy with methotrexate as a single agent appears to produce clinical remission in one half of the cases treated. Dosing is usually 2.5 to 10 mg daily by mouth for weeks or months. Dose levels of drug and adjustment of dose regimen by reduction or cessation of drug are guided by patient response and hematologic monitoring. **Psoriasis Chemotherapy** The patient should be fully informed of the risks involved and should be under constant supervision of the physician. Assessment of renal function, liver function, and blood elements should be made by history, physical examination, and laboratory tests (such as complete blood count (CBC), urinalysis, serum creatinine, liver function studies, and liver biopsy if indicated) before beginning Methotrexate, periodically during methotrexate therapy, and before reinstituting methotrexate therapy after a rest period. Appropriate steps should be taken to avoid conception during and for at least eight weeks following methotrexate therapy. There are two commonly used general types of dosing schedules: 1. Weekly oral intermittent large doses; 2. Divided dose intermittent oral schedule over a 36-hour period. All schedules should be continually tailored to the individual patient. Dose schedules cited below pertain to an average 70 kg adult. An initial test dose one week prior to initiation of therapy is recommended to detect any idiosyncrasy. Recommended starting dose schedules: 1. Weekly single oral: 10–25 mg per week until adequate response is achieved. With this dosage schedule, 25 mg per week should not ordinarily be exceeded. 2. Divided oral dose schedule: 2.5 mg every 12 hours for three doses each week. With this dosage schedule, 30 mg per week should not be exceeded. Dosages in each schedule may be gradually adjusted to achieve optimal clinical response, but not to exceed the maximum stated for each schedule. Once optimal clinical response has been achieved, the dosing schedule should be reduced to the lowest possible amount of drug and to the longest possible dosing interval. The use of methotrexate may permit the return to conventional topical therapy, which should be encouraged. **Use in elderly** Due to diminished hepatic and renal function as well as decreased folate stores in elderly patients, relatively low doses (especially in psoriasis indications) should be considered and these patients should be closely monitored for early signs of toxicity (See section _**4.4 Special warnings and precautions for use**_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). See Table 1 below for reduced doses in oncology patients. **Use in patients with renal impairment – dose adjustments** Methotrexate is excreted to a significant extent by the kidneys, thus in patients with renal impairment the health care provider may need to adjust the dose to prevent accumulation of drug. The table below provided recommended starting doses in renally impaired patients; dosing may need further adjustment due to wide intersubject pK variability. 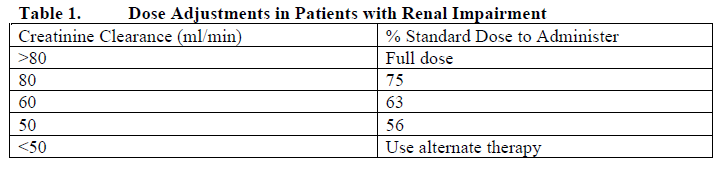 **Folate supplementation** In patients with rheumatoid arthritis, or psoriasis, folic acid or folinic acid may reduce methotrexate toxicities such as gastrointestinal symptoms, stomatitis, alopecia, and elevated liver enzymes. Before taking a folate supplement, it is advisable to check B12 levels, particularly in adults over the age of 50, since folate administration can mask symptoms of B12 deficiency.
ORAL
Medical Information
**4.1 Therapeutic indications** **Anti-neoplastic Chemotherapy** Methotrexate is indicated for the treatment of gestational choriocarcinoma, and in patients with chorioadenoma destruens and hydatidiform mole. Methotrexate is indicated for the palliation of acute lymphocytic leukemia. It is also indicated in the treatment and prophylaxis of meningeal leukemia. Greatest effect has been observed in palliation of acute lymphoblastic (stem-cell) leukemias in children. In combination with other anticancer drugs or suitable agents methotrexate may be used for induction of remission, but it is most commonly used, as described in the literature, in the maintenance of induced remissions. Methotrexate may be used alone or in combination with other anticancer agents in the management of breast cancer, epidermoid cancers of the head and neck, and lung cancer, particularly squamous cells and small cell types. Methotrexate is also effective in the treatment of the advanced stages (III and IV, Peters Staging System) of lymphosarcoma, particularly in those cases in children; and in advanced cases of mycosis fungoides. **Psoriasis Chemotherapy** (See section _**4.4 Special warnings and precautions for use**_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Because of high risk attending its use. Methotrexate is only indicated in the symptomatic control of severe, recalcitrant, disabling psoriasis which is not adequately responsive to other forms of therapy, but only when the diagnosis has been established, as by biopsy and/or after dermatologic consultation.
**4.3 Contraindications** - Hypersensitivity to methotrexate or any excipients in the formulation. - Breast feeding. - Severe renal impairment. _**Applies to patients with psoriasis only:**_ - Alcoholism, alcoholic liver disease, or other chronic liver disease. - Overt or laboratory evidence of immunodeficiency syndromes. - Preexisting blood dyscrasias, such as bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia. - Pregnancy.
L01BA01
methotrexate
Manufacturer Information
PFIZER PRIVATE LIMITED
Excella GmbH & Co. KG
Active Ingredients
Documents
Package Inserts
Methotrexate Tablet PI & PIL.pdf
Approved: August 30, 2022
