Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**III. DOSAGE AND ADMINISTRATION** **The vial of AGGRASTAT (concentrate) must be diluted prior to administration (see INSTRUCTIONS FOR USE).** _AGGRASTAT is for intravenous use only using sterile equipment._ AGGRASTAT may be co‑administered with heparin through the same line. AGGRASTAT is recommended for use with a calibrated infusion device. Care should be taken to avoid a prolonged loading infusion. Care should also be taken in calculating the bolus dose and infusion rates based on patient weight. In clinical studies, patients received aspirin, unless contraindicated. _**Unstable Angina Pectoris or Non-Q-Wave Myocardial Infarction:**_ AGGRASTAT should be administered intravenously, in combination with heparin, at the initial infusion rate of 0.4 microgram/kg/min for 30 minutes. Upon completion of the initial infusion, AGGRASTAT should be continued at a maintenance infusion rate of 0.1 microgram/kg/min. The table below is provided as a guide to dosage adjustment by weight. **AGGRASTAT Injection must first be diluted prior to administration (see INSTRUCTIONS FOR USE).** 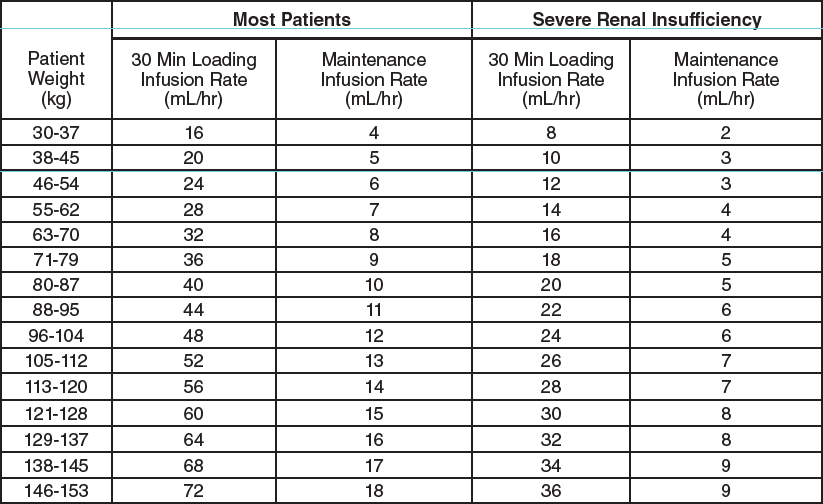 In the study that demonstrated efficacy, AGGRASTAT in combination with heparin was generally continued for a minimum of 48 hours and up to 108 hours; on average patients received AGGRASTAT for 71.3 hours. This infusion can be continued through angiography and should be continued up to 12 to 24 hours postangioplasty/atherectomy. Arterial sheaths should be removed when the patient’s activated clotting time is <180 seconds or 2–6 hours following cessation of heparin. (See CLINICAL PHARMACOLOGY, _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) _**Angioplasty/Atherectomy:**_ In patients in whom AGGRASTAT is initiated in the setting of angioplasty/atherectomy, AGGRASTAT should be administered intravenously, in combination with heparin, as an initial bolus of 10 microgram/kg administered over 3 minutes followed by a maintenance infusion rate of 0.15 microgram/kg/min (see CLINICAL PHARMACOLOGY, _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The table below is provided as a guide to dosage adjustment by weight. **AGGRASTAT Injection must first be diluted prior to administration (see INSTRUCTIONS FOR USE).** 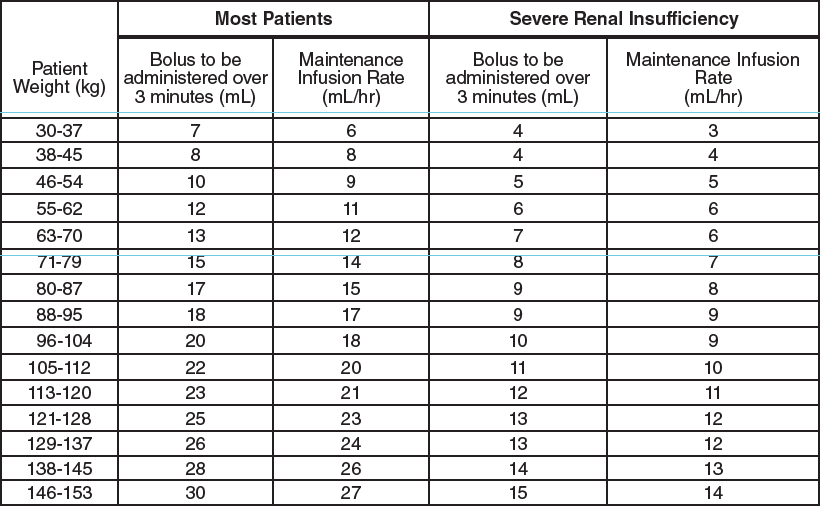 The AGGRASTAT maintenance infusion should be administered for 36 hours. Upon completion of the procedure, heparin should be discontinued and arterial sheaths should then be removed when the patient’s activated clotting time is <180 seconds. _**Patients With Severe Renal Insufficiency**_ As specified in the above dosing tables, the dosage of AGGRASTAT should be decreased by 50% in patients with severe renal insufficiency (creatinine clearance <30 mL/min). (See PRECAUTIONS, _Severe Renal Insufficiency_ and CLINICAL PHARMACOLOGY, _Pharmacokinetics, Characteristics in Patients, Renal Insufficiency – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _._) _**Other Patient Populations**_ No dosage adjustment is recommended for elderly patients (see USE IN THE ELDERLY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) or female patients. **INSTRUCTIONS FOR USE** Parenteral drug products should be inspected visually for particulate matter and discoloration prior to use, whenever solution and container permit. The vial of AGGRASTAT (concentrate) must be diluted prior to administration (see _Directions for Preparation of AGGRASTAT Solution for Infusion from Concentrate_). _Directions for Preparation of AGGRASTAT Solution for Infusion from Concentrate_ 1. Withdraw 50 mL from a 250 mL bag of sterile 0.9% saline or 5% dextrose in water and replace it with 50 mL of AGGRASTAT (from one 50 mL vial) to achieve a concentration of 50 microgram/mL. Mix well before administration. 2. Administer according to the appropriate dosage adjustments by weight above. 3. Any unused intravenous solution should be discarded. AGGRASTAT may be administered in the same intravenous line as atropine sulfate, dobutamine, dopamine, epinephrine HCl, furosemide, lidocaine, midazolam HCl, morphine sulfate, nitroglycerin, potassium chloride, propranolol HCl, and PEPCID\* (famotidine) Injection. AGGRASTAT should not be administered in the same intravenous line as diazepam.
INTRAVENOUS
Medical Information
**II. INDICATIONS** AGGRASTAT, in combination with heparin, is indicated for patients with unstable angina or non-Q-wave myocardial infarction to prevent cardiac ischemic events and is also indicated for patients with coronary ischemic syndromes undergoing coronary angioplasty or atherectomy to prevent cardiac ischemic complications related to abrupt closure of the treated coronary artery. (See CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ and DOSAGE AND ADMINISTRATION.)
**IV. CONTRAINDICATIONS** AGGRASTAT is contraindicated in patients who are hypersensitive to any component of the product. Since inhibition of platelet aggregation increases the risk of bleeding, AGGRASTAT is contraindicated in patients with active internal bleeding; a history of intracranial hemorrhage, intracranial neoplasm, arteriovenous malformation, or aneurysm; and in patients who developed thrombocytopenia following prior exposure to AGGRASTAT.
Pending
xpending
Manufacturer Information
DCH AURIGA SINGAPORE
Patheon Manufacturing Services LLC
Active Ingredients
Documents
Package Inserts
Aggrastat Concentrate for Infusion PI.pdf
Approved: January 28, 2015
