Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION, CONCENTRATE
**2 DOSAGE AND ADMINISTRATION** **2.1 Initial Dosing Information** - Administer BUSULFEX in combination with cyclophosphamide as a conditioning regimen prior to bone marrow or peripheral blood progenitor cell replacement. For adult patients, the recommended doses (BuCY2 regimen) are: - BUSULFEX 0.8 mg/kg (ideal body weight or actual body weight, whichever is lower) intravenously via a central venous catheter as a two-hour infusion every six hours for four consecutive days for a total of 16 doses (Days -7, -6, -5 and -4). - Cyclophosphamide 60 mg/kg intravenously as a one-hour infusion on each of two days beginning no sooner than six hours following the 16th dose of BUSULFEX (Days -3 and -2). - Administer hematopoietic progenitor cells on Day 0. - Premedicate patients with anticonvulsants (e.g., benzodiazepines, phenytoin, valproic acid or levetiracetam) to prevent seizures reported with the use of high dose BUSULFEX. Administer anticonvulsants 12 hours prior to BUSULFEX to 24 hours after the last dose of BUSULFEX. _\[see Warnings and Precautions (5.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Administer antiemetics prior to the first dose of BUSULFEX and continue on a fixed schedule through BUSULFEX administration. - BUSULFEX clearance is best predicted when the BUSULFEX dose is administered based on adjusted ideal body weight. Dosing BUSULFEX based on actual body weight, ideal body weight or other factors can produce significant differences in BUSULFEX clearance among lean, normal and obese patients. - Calculate ideal body weight (IBW) as follows (height in cm, and weight in kg): 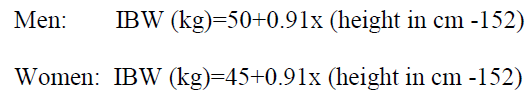 - For obese or severely obese patients, base BUSULFEX dosing on adjusted ideal body weight (AIBW): 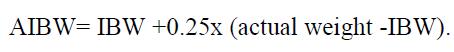 **Pediatrics:** The effectiveness of BUSULFEX in the treatment of CML has not been specifically studied in pediatric patients. For additional information see Special Populations–Pediatric section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **2.2 Preparation and Administration Precautions** **DO NOT USE POLYCARBONATE SYRINGES OR POLYCARBONATE FILTER NEEDLES WITH BUSULFEX.** Use an administration set with minimal residual hold-up volume (2–5 cc) for product administration. BUSULFEX is a cytotoxic drug. Follow applicable special handling and disposal procedures. Skin reactions may occur with accidental exposure. Use gloves when preparing BUSULFEX. If BUSULFEX or diluted BUSULFEX solution contacts the skin or mucosa, wash the skin or mucosa thoroughly with water. Visually inspect parenteral drug products for particulate matter and discoloration prior to administration whenever the solution and container permit. Do not use if particulate matter is seen in the BUSULFEX vial. **2.3 Preparation for Intravenous Administration** BUSULFEX must be diluted prior to intravenous infusion with either 0.9% Sodium Chloride Injection, USP (normal saline) or 5% Dextrose Injection, USP (D5W). The diluent quantity should be 10 times the volume of BUSULFEX, so that the final concentration of busulfan is approximately 0.5 mg/mL. Calculation of the dose for a 70 kg patient would be performed as follows: 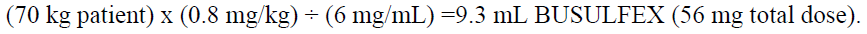 To prepare the final solution for infusion, add 9.3 mL of BUSULFEX to 93 mL of diluent (normal saline or D5W) as calculated below: 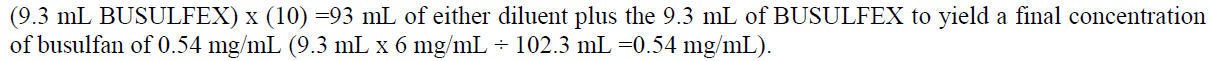 All transfer procedures require strict adherence to aseptic techniques, preferably employing a vertical laminar flow safety hood while wearing gloves and protective clothing. DO NOT put the BUSULFEX into an intravenous bag or large-volume syringe that does not contain normal saline or D5W. Always add the BUSULFEX to the diluent, not the diluent to the BUSULFEX. Mix thoroughly by inverting several times. Infusion pumps should be used to administer the diluted BUSULFEX solution. Set the flow rate of the pump to deliver the entire prescribed BUSULFEX dose over two hours. Prior to and following each infusion, flush the indwelling catheter line with approximately 5 mL of 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. DO NOT infuse concomitantly with another intravenous solution of unknown compatibility. WARNING: RAPID INFUSION OF BUSULFEX HAS NOT BEEN TESTED AND IS NOT RECOMMENDED.
INTRAVENOUS
Medical Information
**1 INDICATIONS AND USAGE** BUSULFEX is indicated for use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic progenitor cell transplantation for chronic myelogenous leukemia.
**4 CONTRAINDICATIONS** BUSULFEX is contraindicated in patients with a history of hypersensitivity to any of its components.
L01AB01
busulfan
Manufacturer Information
STEWARD CROSS PTE LTD
Baxter Oncology GmbH
Active Ingredients
Documents
Package Inserts
Busulfex Injection PI.pdf
Approved: April 6, 2016
