Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**Dosage and Administration** The dose of _LANOXIN_ for each patient has to be tailored individually according to age, lean body weight and renal function. Suggested doses are intended only as an initial guide. The difference in bioavailability between injectable _LANOXIN_ and oral formulations must be considered when changing from one dosage form to another. For example if patients are switched from oral to the i.v. formulation the dosage should be reduced by approximately 33 %. _LANOXIN_ Oral Solution, 50 micrograms in 1 ml, is supplied with a graduated pipette and this should be used for measurement of all doses. **Monitoring** Serum concentrations of _LANOXIN_ may be expressed in Conventional Units of ng/ml or SI Units of nmol/l. To convert ng/ml to nmol/l, multiply ng/ml by 1.28. The serum concentration of digoxin can be determined by radioimmunoassay. Blood should be taken 6 hours or more after the last dose of _LANOXIN_. There are no rigid guidelines as to the range of serum concentrations that are most efficacious. A post hoc analysis of heart failure patients in the Digitalis Investigation Group trial demonstrated that at low serum digoxin concentrations (0.5–0.9 ng/ml), the use of digoxin was associated with reductions in mortality and hospitalisation. Patients with higher digoxin levels (> 1ng/ml) had a higher incidence of morbidity and mortality, although at these concentrations digoxin reduces heart failure hospitalisation. Therefore, the optimal trough digoxin serum level may be 0.5 ng/mL (0.64 nanomol/L) to 1.0 ng/mL (1.28 nanomol/L). _LANOXIN_ toxicity is more commonly associated with serum digoxin concentration greater than 2 ng/mL. However, serum digoxin concentration should be interpreted in the clinical context. Toxicity may occur with lower digoxin serum concentrations. In deciding whether a patient’s symptoms are due to _LANOXIN_, the clinical state together with the serum potassium level and thyroid function are important factors ( _see Overdose_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Other glycosides, including metabolites of digoxin, can interfere with the assays that are available and one should always be wary of values which do not seem commensurate with the clinical state of the patient. **Dilution of _LANOXIN_ Injection** _LANOXIN_ Injection can be administered undiluted or diluted with a 4-fold or greater volume of diluent. The use of less than a 4-fold volume of diluent could lead to precipitation of digoxin. _LANOXIN_ Injection, 250 micrograms per ml when diluted in the ratio of 1 to 250 (i.e. one 2 ml ampoule containing 500 micrograms added to 500 ml of infusion solution) is known to be compatible with the following infusion solutions and stable for up to 48 hours at room temperature (20 to 25 °C). - Sodium Chloride Intravenous Infusion, B.P., 0.9 % w/v - Sodium Chloride (0.18 % w/v) and Glucose (4 % w/v) Intravenous Infusion, B.P. - Glucose Intravenous Infusion, B.P., 5 % w/v. Dilution should be carried out either under full aseptic conditions or immediately before use. Any unused solution should be discarded. **Populations** **• Adults and children over 10 years** **_Parenteral Loading_** NOTE: For use in patients who have not been given cardiac glycosides within the preceding two weeks. The total loading dose of parenteral _LANOXIN_ is 500 to 1000 micrograms (0.5 to 1.0 mg) depending on age, lean body weight and renal function. The total loading dose should be administered in divided doses with approximately half of the total dose given as the first dose and further fractions of the total dose given at intervals of 4 to 8 hours. An assessment of clinical response should be performed before giving each additional dose. Each dose should be given by intravenous infusion ( _see Dilution of LANOXIN Injection above_) over 10 to 20 minutes. **_Rapid Oral Loading_** If medically appropriate, rapid digitalisation may be achieved in a number of ways, such as the following: 750 to 1500 micrograms (0.75 to 1.5 mg) as a single dose. Where there is less urgency, or greater risk of toxicity, e.g. in the elderly, the oral loading dose should be given in divided doses 6 hours apart, with approximately half the total dose given as the first dose. Clinical response should be assessed before giving each additional dose ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_Slow Oral Loading_** In some patients, for example those with mild heart failure, digitalisation may be achieved more slowly with doses of 250 to 750 micrograms (0.25 to 0.75 mg) daily for 1 week followed by an appropriate maintenance dose. A clinical response should be seen within one week.  **_Maintenance Dose:_** The maintenance dosage should be based upon the percentage of the peak body stores lost each day through elimination. The following formula has had wide clinical use. 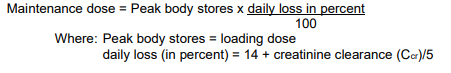 Ccr is creatinine clearance corrected to 70 kg bodyweight or 1.73 m2 body surface area. If only serum creatinine (Scr) concentrations are available, a Ccr (corrected to 70 kg bodyweight) may be estimated in men as: 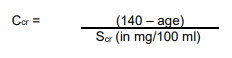 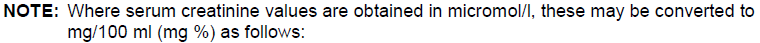  **For women**, this result should be multiplied by 0.85. **Note: These formulae cannot be used for creatinine clearance in children.** In practice, this will mean that most patients with heart failure will be maintained on 125 to 250 micrograms (0.125 to 0.25 mg) _LANOXIN_ daily; however in those who show increased sensitivity to the adverse effects of _LANOXIN_, a dose of 62.5 micrograms (0.0625 mg) daily or less may suffice. Conversely, some patients may require a higher dose. **• Neonates, infants and children up to 10 years of age** (if cardiac glycosides have not been given in the preceding two weeks) If cardiac glycosides have been given in the two weeks preceding commencement of digoxin therapy, it should be anticipated that optimum loading doses of digoxin will be less than those recommended below. In the newborn, particularly in the premature infant, renal clearance of _LANOXIN_ is diminished and suitable dose reductions must be observed, over and above general dosage instructions. Beyond the immediate newborn period, children generally require proportionally larger doses than adults on the basis of body weight or body surface area, as indicated in the schedule below. Children over 10 years of age require adult dosages in proportion to their body weight. **_Parenteral Loading Dose_** The intravenous loading dose in the above groups should be administered in accordance with the following schedule. Preterm neonates <1.5 kg– 20 micrograms /kg over 24 hours.Preterm neonates 1.5 kg–2.5 kg– 30 micrograms /kg over 24 hours.Term neonates to 2 years– 35 micrograms /kg over 24 hours.2 to 5 years– 35 micrograms /kg over 24 hours.5 to 10 years– 25 micrograms /kg over 24 hours. The loading dose should be administered in divided doses with approximately half the total dose given as the first dose and further fractions of the total dose given at intervals of 4 to 8 hours, assessing clinical response before giving each additional dose. Each dose should be given by intravenous infusion ( _see Dilution of LANOXIN Injection above_) over 10 to 20 minutes. **_Oral Loading Dose_** This should be administered in accordance with the following schedule. Preterm neonates <1.5 kg– 25 micrograms /kg per 24 hours.Preterm neonates 1.5 kg to 2.5 kg– 30 micrograms /kg per 24 hours.Term neonates to 2 years– 45 micrograms /kg per 24 hours.2 to 5 years– 35 micrograms /kg per 24 hours.5 to 10 years– 25 micrograms /kg per 24 hours. The loading dose should be administered in divided doses with approximately half the total dose given as the first dose and further fractions of the total dose given at intervals of 4 to 8 hours, assessing clinical response before giving each additional dose. **_Maintenance_** The maintenance dose should be administered in accordance with the following schedule. **Preterm neonates:** daily dose = 20 % of 24-hour loading dose (i.v. or oral). **Term neonates and children up to 10 years:** daily dose = 25 % of 24-hour loading dose (i.v. or oral). These dosage schedules are meant as guidelines and careful clinical observation and monitoring of serum _LANOXIN_ levels ( _see Monitoring above_) should be used as a basis for adjustment of dosage in these paediatric patient groups. **• Elderly** The tendency to impaired renal function and low lean body mass in the elderly influences the pharmacokinetics of _LANOXIN_ such that high serum digoxin levels and associated toxicity can occur quite readily, unless doses of _LANOXIN_ lower than those in non-elderly patients are used. Serum digoxin levels should be checked regularly and hypokalaemia avoided. **• Dose Recommendations in Specific Patients Groups** _See Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
ORAL
Medical Information
**Indications** **Cardiac Failure** _LANOXIN_ is indicated in the management of chronic cardiac failure where the dominant problem is systolic dysfunction. Its therapeutic benefit is greatest in those patients with ventricular dilatation. _LANOXIN_ is specifically indicated where cardiac failure is accompanied by atrial fibrillation. **Supraventricular Arrhythmias** _LANOXIN_ is indicated in the management of certain supraventricular arrhythmias, particularly chronic atrial flutter and fibrillation.
**Contraindications** _LANOXIN_ is contraindicated in intermittent complete heart block or second degree atrioventricular block, especially if there is a history of Stokes-Adams attacks. _LANOXIN_ is contraindicated in arrhythmias caused by cardiac glycoside intoxication. _LANOXIN_ is contraindicated in supraventricular arrhythmias associated with an accessory atrioventricular pathway, as in the Wolff-Parkinson-White syndrome, unless the electrophysiological characteristics of the accessory pathway and any possible deleterious effect of _LANOXIN_ on these characteristics have been evaluated. If an accessory pathway is known or suspected to be present and there is no history of previous supraventricular arrhythmias, _LANOXIN_ is similarly contraindicated. _LANOXIN_ is contraindicated in ventricular tachycardia or ventricular fibrillation. _LANOXIN_ is contraindicated in hypertrophic obstructive cardiomyopathy, unless there is concomitant atrial fibrillation and heart failure but even then caution should be exercised if _LANOXIN_ is to be used. _LANOXIN_ is contraindicated in patients known to be hypersensitive to digoxin, other digitalis glycosides or to any component of the preparation.
C01AA05
digoxin
Manufacturer Information
DCH AURIGA SINGAPORE
ASPEN BAD OLDESLOE GMBH
Active Ingredients
Documents
Package Inserts
Digoxin PI.pdf
Approved: March 19, 2020
