Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**DOSAGE AND ADMINISTRATION** **Treatment of shingles (herpes zoster)** The dosage in adults is 1000 mg three times a day for 7 days. **Treatment of herpes simplex infections** The dosage in adults is 500 mg of Valacyclovir twice daily. For recurrent infections, treatment should be maintained for 5 days. For initial episodes, which can be more severe, treatment may have to be extended to 10 days. Dosing should begin as early as possible. For recurrent episodes of herpes simplex, this should ideally be during the prodromal period or as soon as the first signs appear. Prophylaxis of recurrences of herpes simplex infections Immunocompetent patients should take 500 mg of Valacyclovir once daily. For immunocompromised adult patients the dose is 500 mg twice daily. There are no data on the reduction of transmission in other patient populations. - **Children** There are no data available on the use of Valaciclovir in children. - **Elderly** The possibility of renal impairment in the elderly must be considered and the dosage should be adjusted accordingly (see Renal impairment below). Adequate hydration should be maintained. - **Renal impairment** Caution is advised when administering valaciclovir to patients with impaired renal function. Adequate hydration should be maintained. The dosage of Valaciclovir should be reduced in patients with impaired renal function as shown in the table below: 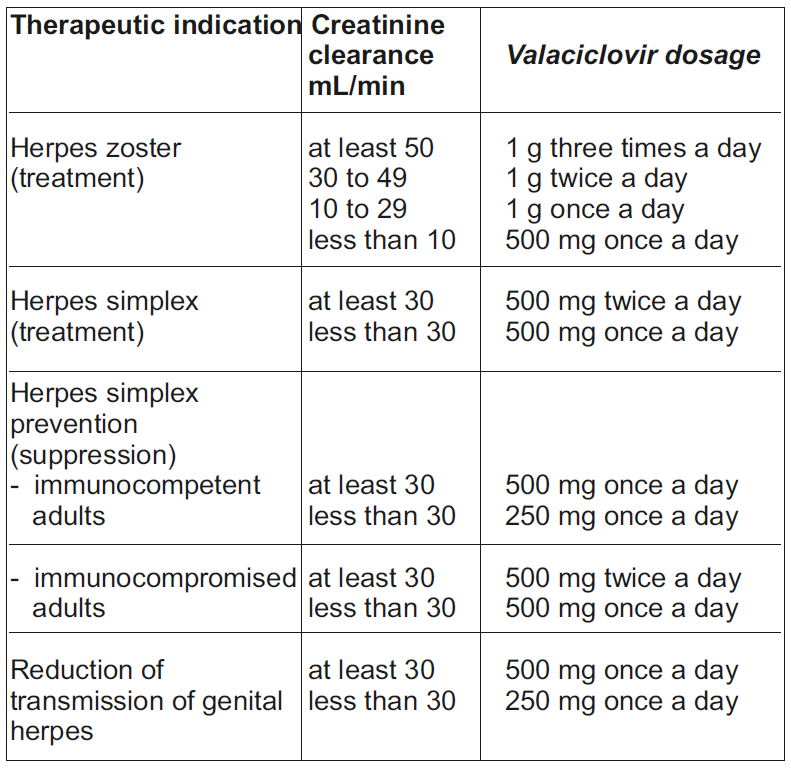 In patients on intermittent haemodialysis, the Valaciclovir dosage should be administered after the haemodialysis has been performed. The creatinine clearance should be monitored frequently, especially during periods when renal function is changing rapidly, the Valaciclovir dosage should be adjusted accordingly. **Hepatic impairment** Studies with a 1 g unit dose of Valaciclovir show that dose modification is not required in patients with mild or moderate cirrhosis (hepatic synthetic function maintained). Pharmacokinetic data in patients with advanced cirrhosis, (impaired hepatic synthetic function and evidence of portal-systemic shunting) do not indicate the need for dosage adjustment; however, clinical experience is limited. For higher doses (4 g or more/day), see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
ORAL
Medical Information
**INDICATIONS** **Treatment of shingles (herpes zoster) infection.** **Herpes simplex infections of the skin and mucous membranes, including initial and recurrent genital herpes.** **Prophylaxis of recurrent herpes simplex infections of the skin and mucous membranes, including genital herpes.**
**CONTRAINDICATIONS** Hypersensitivity to Valacyclovir, acyclovir or any of the excipients.
J05AB11
valaciclovir
Manufacturer Information
MEDICELL PHARMACEUTICAL (S) PTE. LTD.
Hetero Labs Limited
Active Ingredients
Documents
Package Inserts
Valacyclovir PI.pdf
Approved: April 26, 2023
