Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**DOSAGE AND ADMINISTRATION** The usual dose of **CRAVIT** ® Tablets is 250 mg or 500 mg administered orally, as indicated by infection and described in the following dosing chart. These recommendations apply to patients with normal renal function (i.e., creatinine clearance > 80 mL/min). For patients with altered renal function see the **Patients with Impaired Renal Function** subsection. Oral doses should be administered at least two hours before or two hours after antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or Videx® (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution. 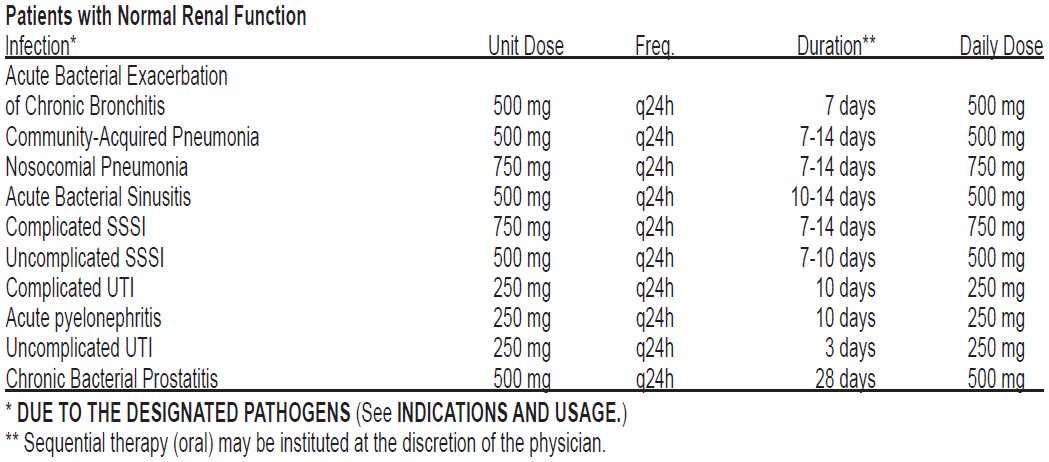 **Patients with Impaired Renal Function**Renal StatusInitial DoseSubsequent Dose**Acute Bacterial Exacerbation of Chronic Bronchitis /** **Comm. Acquired Pneumonia / Acute Maxillary Sinusitis / Uncomplicated SSSI**CLCR from 50 to 80 mL/minNo dosage adjustment requiredCLCR from 20 to 49 mL/min500 mg250 mg q24hCLCR from 10 to 19 mL/min500 mg250 mg q48hHemodialysis500 mg250 mg q48hCAPD500 mg250 mg q48h**Complicated SSSI**CLCR from 50 to 80 mL/minNo dosage adjustment requiredCLCR from 20 to 49 mL/min750 mg750 mg q48hCLCR from 10 to 19 mL/min750 mg500 mg q48hHemodialysis750 mg500 mg q48hCAPD750 mg500 mg q48h**Complicated UTI / Acute Pyelonephritis**CLCR ≥20 mL/minNo dosage adjustment requiredCLCR from 10 to 19 mL/min250 mg250 mg q48h**Uncomplicated UTI**No dosage adjustment requiredCLCR = creatinine clearancesCAPD = chronic ambulatory peritoneal dialysis When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance.  The serum creatinine should represent a steady state of renal function.
ORAL
Medical Information
**INDICATIONS AND USAGE** **CRAVIT** ® Tablets are indicated for the treatment of adults (≥18 years of age) with mild, moderate, and severe infections caused by susceptible strains of the designated microorganisms in the conditions listed below. Please see **DOSAGE AND ADMINISTRATION** for specific recommendations. **Acute bacterial sinusitis** due to _Streptococcus pneumoniae, Haemophilus influenzae,_ or _Moraxella catarrhalis_. **Acute bacterial exacerbation of chronic bronchitis** due to _Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae,_ or _Moraxella catarrhalis_. **Community-acquired pneumonia** due to _Staphylococcus aureus, Streptococcus pneumoniae_ (including penicillin-resistant strains, MIC value for penicillin ≥2mcg/mL), _Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydia pneumoniae, Legionella pneumophila,_ or _Mycoplasma pneumoniae_. (See **CLINICAL STUDIES** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) **Nosocomial pneumonia** due to methicillin-susceptible _Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, Streptococcus pneumoniae_. Adjunctive therapy should be used as clinically indicated. Where _Pseudomonas aeruginosa_ is a documented or presumptive pathogen, combination therapy with an anti-pseudomonal β-lactam is recommended. **Complicated skin and skin structure infections** due to methicillin-sensitive _Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes,_ or _Proteus mirabilis_. **Uncomplicated skin and skin structure infections** (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections due to _Staphylococcus aureus,_ or _Streptococcus pyogenes_. **Complicated urinary tract infections** (mild to moderate) due to _Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis,_ or _Pseudomonas aeruginosa_. **Acute pyelonephritis** (mild to moderate) caused by _Escherichia coli_. **Uncomplicated urinary tract infections** (mild to moderate) due to _Escherichia coli, Klebsiella pneumoniae_, or _Staphylococcus saprophyticus_. **Chronic bacterial prostatitis** due to _Escherichia coli, Enterococcus faecalis_, or methicillin-susceptible _Staphylococcus epidermidis_. Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin. Therapy with levofloxacin may be initiated before results of these tests are known; once results become available, appropriate therapy should be selected. As with other drugs in this class, some strains of _Pseudomonas aeruginosa_ may develop resistance fairly rapidly during treatment with levofloxacin. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance.
**CONTRAINDICATIONS** Levofloxacin is contraindicated in persons with a history of hypersensitivity to levofloxacin, quinolone antimicrobial agents, or any other components of this product.
J01MA12
levofloxacin
Manufacturer Information
RANBAXY (MALAYSIA) SDN. BHD.
PT KALBE FARMA TBK
Active Ingredients
Documents
Package Inserts
Cravit Tablet PI.pdf
Approved: December 30, 2021
