Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** Pharmaceutical Form: Coated tablet. The usual course of therapy is seven days (range 5 – 10 days). _ZINNAT_ should be taken after food for optimum absorption. **Dosage in adults:**  **Dosage in children:**  _ZINNAT_ tablets should not be crushed or split and are therefore unsuitable for treatment of patients, such as younger children, who cannot swallow whole tablets. Therefore, _ZINNAT_ suspension is recommended for patients who cannot swallow whole tablets. When doses below 250 mg are required, _ZINNAT_ FOR SUSPENSION 125mg/5ml should be used. There is no experience of using _ZINNAT_ in children under the age of 3 months. **Dosage in renal impairment:** Cefuroxime is primarily excreted by the kidneys. In patients with markedly impaired renal function, it is recommended that the dosage of cefuroxime be reduced to compensate for its slower excretion (see the table below). 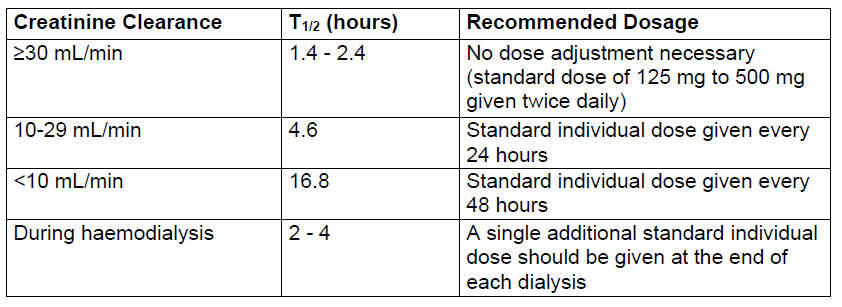
ORAL
Medical Information
**Indications** _ZINNAT_ is an oral prodrug of the bactericidal cephalosporin antibiotic cefuroxime, which is resistant to most β (beta)-lactamases and is active against a wide range of Gram-positive and Gram-negative organisms. It is indicated for the treatment of infections caused by susceptible bacteria. Susceptibility to _ZINNAT_ will vary with geography and time, and it should be used in accordance with local official antibiotic prescribing guidelines and local susceptibility data (see _Pharmacological properties_, _Pharmacodynamics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Indications include:** Upper respiratory tract infections for example, ear, nose and throat infections, such as otitis media, sinusitis, tonsillitis and pharyngitis. Lower respiratory tract infections for example, pneumonia and acute exacerbations of chronic bronchitis. Genito-urinary tract infections for example, pyelonephritis, cystitis and urethritis. Skin and soft tissue infections for example, furunculosis, pyoderma and impetigo. Gonorrhoea, acute uncomplicated gonococcal urethritis and cervicitis.
**Contraindications** Patients with known hypersensitivity to cephalosporin antibiotics.
J01DC02
cefuroxime
Manufacturer Information
SANDOZ SINGAPORE PTE. LTD.
Glaxo Operations UK Limited (trading as Glaxo Wellcome Operations)
Active Ingredients
Documents
Package Inserts
Zinnat Tablet PI.pdf
Approved: November 4, 2022
