Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**DOSAGE AND ADMINISTRATION** SUPRANE (desflurane) is administered by inhalation. The concentration of SUPRANE (desflurane) should be delivered from a vaporiser specifically designed and designated for use with SUPRANE (desflurane). The administration of general anaesthesia must be individualised based on the patient’s response. Opioids or benzodiazepines decrease the amounts of SUPRANE (desflurane) required to produce anaesthesia. SUPRANE (desflurane) decreases the required doses of neuromuscular blocking agents. (See **Table 2** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information._) If added relaxation is required, supplemental doses of muscle relaxants may be used. (See **INTERACTION WITH OTHER MEDICAMENTS AND OTHER FORMS OF INTERACTION** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information._) **Dosage** The minimum alveolar concentration (MAC) of SUPRANE (desflurane) decreases with increasing patient age. The dose of SUPRANE (desflurane) should be adjusted accordingly. The MAC has been determined as listed in Table 1. 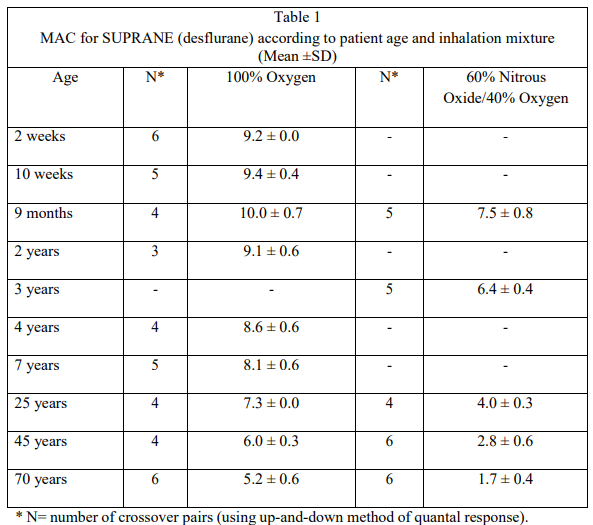 In patients with coronary artery disease, maintenance of normal haemodynamics is important for avoidance of myocardial ischaemia. SUPRANE (desflurane) should not be used as the sole agent for anaesthetic induction in patients at risk of coronary artery disease or in patients where increases in heart rate or blood pressure are undesirable. It should be used with other medications, preferably intravenous opioids and hypnotics. **Premedication** Issues such as whether or not to premedicate and the choice of premedicant(s) must be individualised. In clinical trials, patients scheduled to be anaesthetised with desflurane frequently received IV pre-anaesthetic medication, such as opioids and/or benzodiazepines. **Induction of Anaesthesia in Adults** In adults, a starting concentration of 3% is recommended, increased in 0.5–1.0% increments every 2 to 3 breaths. Inspired concentrations of 4–11% SUPRANE (desflurane) produce surgical anaesthesia within 2 to 4 minutes. Higher concentrations up to 15% may be used. Such concentrations of SUPRANE (desflurane) will proportionately dilute the concentration of oxygen and commencing administration of oxygen should be 30% or above. During induction in adults, the overall incidence of oxyhaemoglobin desaturation (SpO2 < 90%) was 6%. High concentrations of SUPRANE (desflurane) may induce upper airway adverse events. (See **ADVERSE REACTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) After induction in adults with an intravenous drug such as thiopental or propofol, SUPRANE (desflurane) can be started at approximately 0.5–1 MAC, whether the carrier gas is oxygen or nitrous oxide/oxygen. SUPRANE (desflurane) should be administered at 0.8 MAC or less, and in conjunction with a barbiturate induction and hyperventilation (hypocapnia) until cerebral decompression in patients with known or suspected increases in cerebrospinal fluid pressure (CSFP). Appropriate attention must be paid to maintain cerebral perfusion pressure. (See **SPECIAL WARNINGS AND PRECAUTIONS FOR USE** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) **Induction of Anaesthesia in Children** SUPRANE (desflurane) is not indicated for use as an inhalation induction agent in children and infants because of the frequent occurrence of cough, breath holding, apnoea, laryngospasm and increase in secretions. **Maintenance of Anaesthesia in Adults** SUPRANE (desflurane) at 2.5–8.5% may be required when administered using oxygen or oxygen enriched air. In adults, surgical levels of anaesthesia may be sustained at a reduced concentration of SUPRANE (desflurane) when nitrous oxide is used concomitantly. **Maintenance of Anaesthesia in Children** SUPRANE (desflurane) is indicated for maintenance of anaesthesia in infants and children. Surgical levels of anaesthesia may be maintained in children with end-tidal concentrations of 5.2 to 10% SUPRANE (desflurane) with or without the concomitant use of nitrous oxide. Although concentrations of up to 18% desflurane have been administered for short periods of time, if high concentrations are used with nitrous oxide it is important to ensure that the inspired mixture contains a minimum of 25% oxygen. **Blood Pressure and Heart Rate During Maintenance** Blood pressure and heart rate should be monitored carefully during maintenance as part of the evaluation of depth of anaesthesia. **Dosage in Renal and Hepatic Impairment** Concentrations of 1–4% SUPRANE (desflurane) in nitrous oxide/oxygen have been used in patients with chronic renal or hepatic impairment and during renal transplantation surgery. Because of minimal metabolism, a need for dose adjustment in patients with renal and hepatic impairment is not to be expected. **Method of Administration** SUPRANE (desflurane) should only be administered by persons trained in the administration of general anaesthesia using a vaporiser specifically designed and designated for use with SUPRANE (desflurane).
NASAL
Medical Information
**INDICATIONS** SUPRANE (desflurane) is indicated as an inhalation agent for induction and/or maintenance of anaesthesia for inpatient and outpatient in adults and maintenance of anaesthesia in infants and children.
**CONTRAINDICATIONS** SUPRANE (desflurane) is contraindicated in patients: - in whom general anaesthesia is contraindicated. - with known sensitivity to halogenated agents. - with a known or suspected genetic susceptibility to malignant hyperthermia. - with a history of malignant hyperthermia, or in whom liver dysfunction, jaundice or unexplained fever, leucocytosis, or eosinophilia has occurred after a previous halogenated anaesthetic administration. - as an inhalation agent in paediatric patients because of the frequent occurrence of cough, breath holding, apnoea, laryngospasm and increased secretions.
N01AB07
desflurane
Manufacturer Information
BAXTER HEALTHCARE (ASIA) PTE LTD
Baxter Healthcare Corporation
Active Ingredients
Documents
Package Inserts
Suprane_PI.pdf
Approved: January 26, 2023
