Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**2.2 Posology and method of administration** General Perjeta is subject to restricted medical prescription and therapy should only be initiated under the supervision of a physician experienced in the administration of anti-cancer agents. Perjeta should be administered by a healthcare professional prepared to manage anaphylaxis and in an environment where full resuscitation service is immediately available. Patients treated with Perjeta must have HER2-positive tumour status, defined as a score of 3+ by immunohistochemistry (IHC) and/or a ratio of ≥ 2.0 by in situ hybridisation (ISH) assessed by a validated test. To ensure accurate and reproducible results, the testing must be performed in a specialised laboratory, which can ensure validation of the testing procedures. For full instructions on assay performance and interpretation please refer to the package leaflets of validated HER2 testing assays. Posology _Metastatic and Early Breast Cancer_ The recommended initial loading dose of Perjeta is 840 mg administered as a 60-minute intravenous infusion, followed every 3 weeks thereafter by a maintenance dose of 420 mg administered over a period of 30 to 60 minutes. An observation period of 30–60 minutes is recommended after completion of each Perjeta infusion. The observation period should be completed prior to any subsequent dose of Herceptin or chemotherapy (see section 2.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Perjeta and Herceptin should be administered sequentially and can be given in any order. When administered with Perjeta, the recommendation is to follow a 3-weekly schedule for Herceptin administered either as: - an IV infusion with an initial dose of 8 mg/kg followed every 3 weeks thereafter by a dose of 6 mg/kg body weight. _or_ - a fixed dose of Herceptin subcutaneous (SC) injection (600 mg) for the initial dose and every 3 weeks thereafter irrespective of the patient’s body weight. In patients receiving a taxane, Perjeta and Herceptin should be administered prior to the taxane. When administered with Perjeta the recommended initial dose of docetaxel is 75 mg/m2, and administered thereafter on a 3-weekly schedule. The dose of docetaxel may be escalated to 100 mg/m2 on subsequent cycles if the initial dose is well tolerated. In patients receiving an anthracycline-based regimen, Perjeta and Herceptin should be administered following completion of the entire anthracycline regimen. _Metastatic Breast Cancer (MBC)_ Perjeta should be administered in combination with Herceptin and docetaxel until disease progression or unmanageable toxicity. Treatment with Perjeta and Herceptin may continue even if treatment with docetaxel is discontinued. _Early Breast Cancer (EBC)_ In the neoadjuvant setting (before surgery), it is recommended that patients are treated with Perjeta for three to six cycles depending on the regimen chosen in combination with Herceptin and chemotherapy (see section 3.1.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In the adjuvant setting, Perjeta should be administered in combination with Herceptin for a total of one year (up to 18 cycles or until disease recurrence, or unmanageable toxicity, whichever occurs first), as part of a complete regimen for early breast cancer and regardless of the timing of surgery. Treatment should include standard anthracycline and/or taxane-based chemotherapy. Perjeta and Herceptin should start on Day 1 of the first taxane-containing cycle and should continue even if chemotherapy is discontinued (see section 3.1.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Delayed or missed doses_ For recommendations on delayed or missed doses, please refer to Table 1 below. 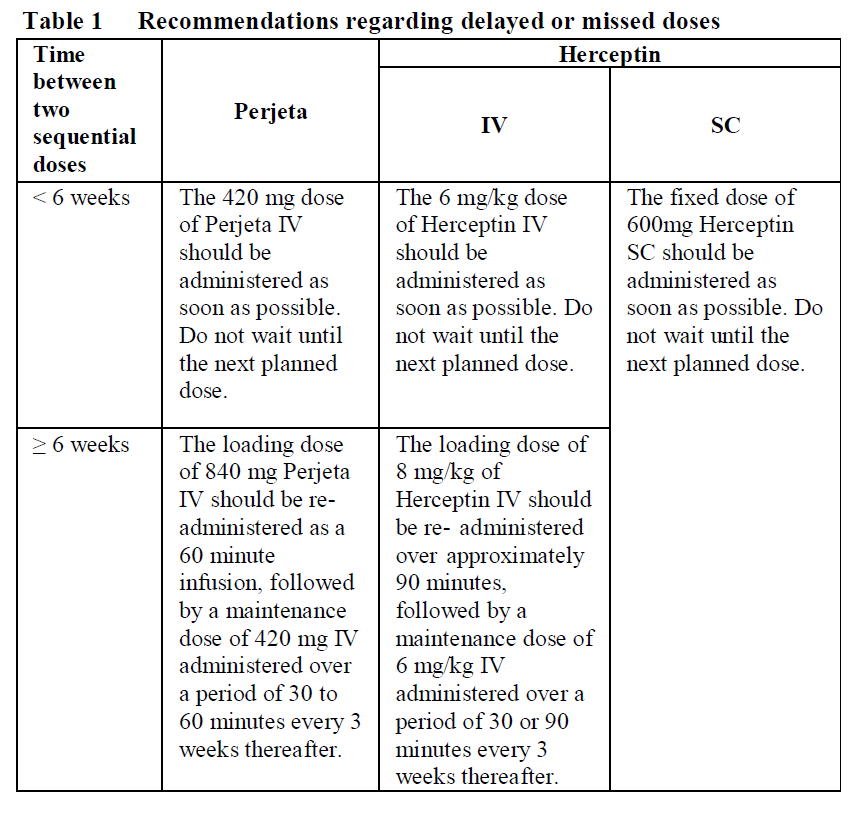 _Dose modifications_ Perjeta should be discontinued if Herceptin treatment is discontinued. Dose reductions are not recommended for Perjeta and Herceptin (see Herceptin prescribing information). Patients may continue therapy during periods of reversible chemotherapy-induced myelosuppression but they should be monitored carefully for complications of neutropenia during this time. For chemotherapy dose modifications, see relevant prescribing information. _Left ventricular dysfunction_ Assess LVEF prior to initiation of Perjeta and at regular intervals during treatment to ensure that LVEF is within normal limits (see Table 2 below). If the LVEF declines as indicated in Table 2 and has not improved, or has declined further at the subsequent assessment, discontinuation of Perjeta and Herceptin should be strongly considered, unless the benefits for the individual patient are deemed to outweigh the risks. 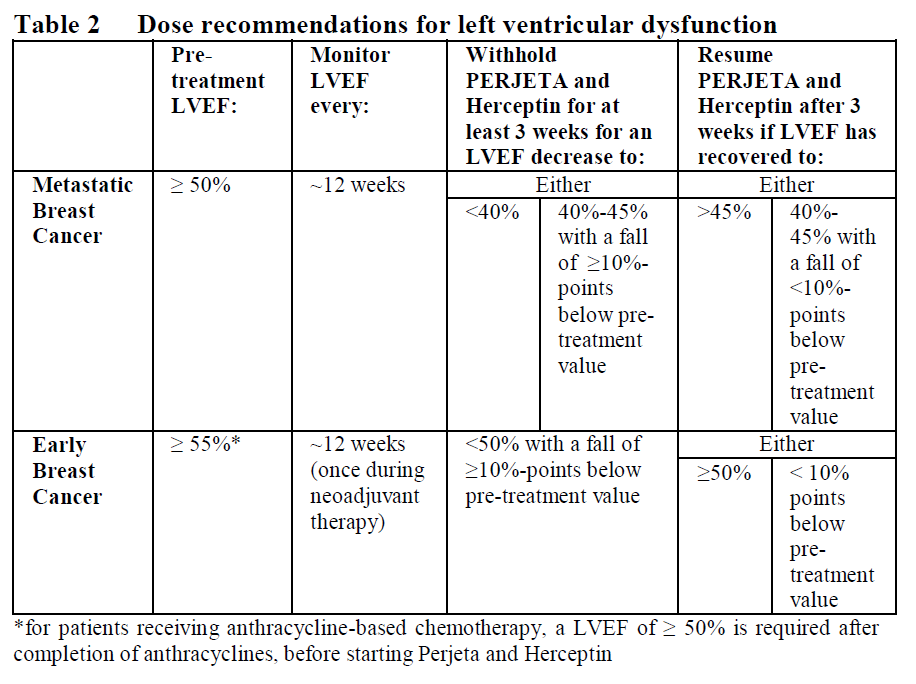 _Infusion reactions_ The infusion rate of Perjeta may be slowed or the administration interrupted if the patient develops an infusion reaction (see section 2.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The infusion may be resumed when symptoms abate. Treatment including oxygen, beta agonists, antihistamines, rapid i.v. fluids and antipyretics may also help alleviate symptoms. The infusion should be discontinued immediately and permanently if the patient experiences a NCI-CTCAE Grade 4 reaction (anaphylaxis), bronchospasm or acute respiratory distress syndrome (see section 2.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Elderly patients_ Limited data are available on the safety and efficacy of pertuzumab in patients ≥ 65 years of age. No dose adjustment is required in patients ≥ 65 years of age (see section 2.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Very limited data are available in patients > 75 years of age. _Patients with renal impairment_ Dose adjustments of Perjeta are not needed in patients with mild or moderate renal impairment. No dose recommendations can be made for patients with severe renal impairment because of the limited pharmacokinetic data available (see section 3.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients with hepatic impairment_ The safety and efficacy of Perjeta have not been studied in patients with hepatic impairment. No specific dose recommendations can be made. _Paediatric population_ The safety and efficacy of Perjeta in children and adolescents below 18 years of age have not been established. There is no relevant use of Perjeta in the paediatric population in the indication of metastatic breast cancer. Method of administration Perjeta is administered intravenously by infusion. It should not be administered as an intravenous push or bolus. For instructions on dilution of Perjeta prior to administration, see section 4.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For the initial dose, the recommended infusion period is 60 minutes. If the first infusion is well tolerated, subsequent infusions may be administered over a period of 30 minutes to 60 minutes (see section 2.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**2.1 Therapeutic indications** Metastatic Breast Cancer (MBC) Perjeta is indicated for use in combination with Herceptin and docetaxel for the treatment of adult patients with HER2-positive metastatic or locally recurrent unresectable breast cancer, who have not received previous anti-HER2 therapy or chemotherapy for their metastatic disease. Early Breast Cancer Perjeta is indicated for use in combination with Herceptin and chemotherapy for the: - neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer. This indication is based on demonstration of an improvement in pathological complete response rate. No data are available demonstrating improvement in event-free survival or overall survival. - adjuvant treatment of patients with HER2-positive early breast cancer at high risk of recurrence (see section 3.1.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**2.3 Contraindications** Hypersensitivity to pertuzumab or to any of the excipients listed in section 4.3 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01XC13
xl 01 xc 13
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Roche Diagnostics GmbH
Active Ingredients
Documents
Package Inserts
Perjeta Concentrate for Solution for Infusion PI.pdf
Approved: April 26, 2021
