Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**2 DOSAGE AND ADMINISTRATION** **2.1 Important Administration Instructions** DUPIXENT is administered by subcutaneous injection. DUPIXENT is intended for use under the guidance of a healthcare provider. Provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use according to the “Instructions for Use”. Use of Pre-Filled Syringe The DUPIXENT pre-filled syringe is for use in adult and pediatric patients aged 6 months and older. A caregiver or patient 12 years of age and older may inject DUPIXENT using the pre-filled syringe. In pediatric patients 12 to 17 years of age, administer DUPIXENT under the supervision of an adult. In pediatric patients 6 months to less than 12 years of age, administer DUPIXENT pre-filled syringe by a caregiver. Administration Instructions For atopic dermatitis, asthma and prurigo nodularis patients taking an initial 600 mg dose, administer each of the two DUPIXENT 300 mg injections at different injection sites. For atopic dermatitis and asthma patients taking an initial 400 mg dose, administer each of the two DUPIXENT 200 mg injections at different injection sites. Administer subcutaneous injection into the thigh or abdomen, except for the 2 inches (5 cm) around the navel. The upper arm can also be used if a caregiver administers the injection. Rotate the injection site with each injection. DO NOT inject DUPIXENT into skin that is tender, damaged, bruised, or scarred. The DUPIXENT “Instructions for Use” contains more detailed instructions on the preparation and administration of DUPIXENT _\[see Instructions for Use\]_. **2.2 Vaccination Prior to Treatment** Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with DUPIXENT _\[see Warnings and Precautions (5.9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.3 Recommended Dosage for Atopic Dermatitis** Dosage in Adults The recommended dosage of DUPIXENT for adult patients is an initial dose of 600 mg (two 300 mg injections), followed by 300 mg given every other week (Q2W). Dosage in Pediatric Patients 6 Months to 5 Years of Age The recommended dosage of DUPIXENT for pediatric patients 6 months to 5 years of age is specified in Table 1. 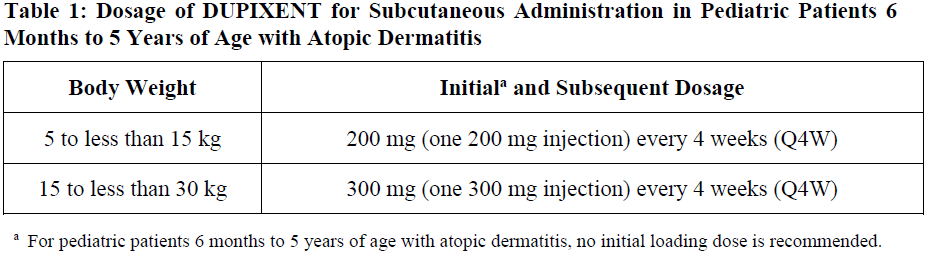 Dosage in Pediatric Patients 6 Years to 17 Years of Age The recommended dosage of Dupixent for pediatric patients 6 years to 17 years of age is specified in Table 2. 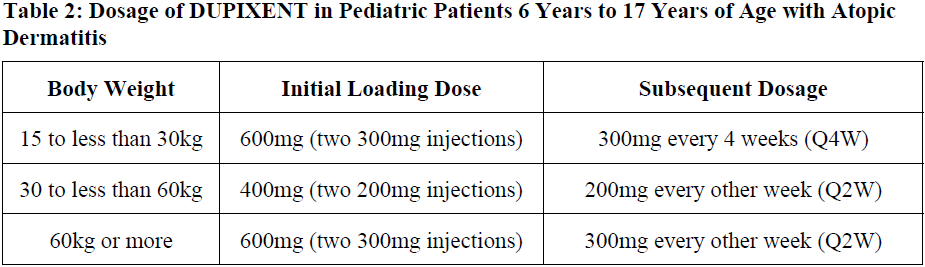 Concomitant Topical Therapies DUPIXENT can be used with or without topical corticosteroids. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. **2.4 Recommended Dosage for Asthma** Adults and Pediatric Patients 12 Years and Older The recommended dosage of DUPIXENT for adults and pediatric patients (12 years of age and older) is: - An initial dose of 400 mg (two 200 mg injections) followed by 200 mg given every other week. - An initial dose of 600 mg (two 300 mg injections) followed by 300 mg given every other week for patients with oral corticosteroids-dependent asthma or with co-morbid moderate-to-severe atopic dermatitis or adults with co-morbid severe chronic rhinosinusitis with nasal polyposis for which DUPIXENT is indicated. Pediatric Patients (6 to 11 years of age) The recommended dosage of DUPIXENT for pediatric patients 6 to 11 years of age is specified in Table 3. 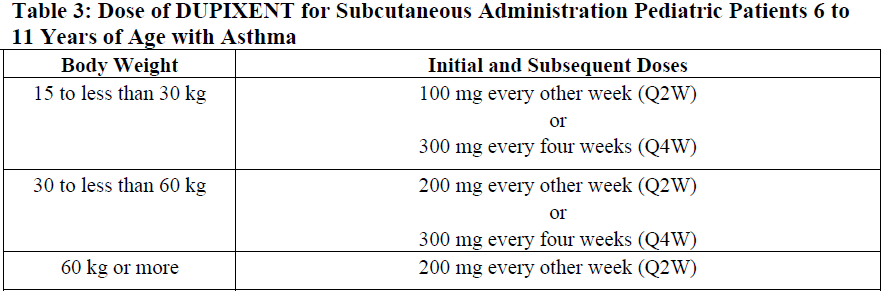 For pediatric patients (6–11 years old) with asthma and co-morbid moderate-to-severe atopic dermatitis, the recommended dosage should be followed in Table 2. **2.5 Recommended Dosage for Chronic Rhinosinusitis with Nasal Polyposis** The recommended dosage of DUPIXENT for adult patients is one DUPIXENT 300mg injection given every other week. **2.6 Recommended Dosage for Prurigo Nodularis** The recommended dosage of DUPIXENT for adult patients is an initial dose of 600mg (two 300mg injections), followed by 300mg given every other week. **2.7 Missed Doses** If an every other week dose is missed, administer the injection within 7 days from the missed dose and then resume the patient's original schedule. If the missed dose is not administered within 7 days, wait until the next dose on the original schedule. If an every 4 week dose is missed, administer the injection within 7 days from the missed dose and then resume the patient's original schedule. If the missed dose is not administered within 7 days, administer the dose, starting a new schedule based on this date. **2.8 Preparation for Use** Before injection, remove DUPIXENT pre-filled syringe from the refrigerator and allow DUPIXENT to reach room temperature (45 minutes for the 300mg/2mL pre-filled syringe, 30 minutes for the 200mg/1.14mL pre-filled syringe and 30 minutes for the 100 mg/0.67mL pre-filled syringe) without removing the needle cap. After removal from the refrigerator, DUPIXENT must be used within 14 days or discarded. Inspect DUPIXENT visually for particulate matter and discoloration prior to administration. DUPIXENT is a clear to slightly opalescent, colorless to pale yellow solution. Do not use if the liquid contains visible particulate matter, is discolored or cloudy (other than clear to slightly opalescent, colorless to pale yellow). DUPIXENT does not contain preservatives; therefore, discard any unused product remaining in the pre-filled syringe.
SUBCUTANEOUS
Medical Information
**1 INDICATIONS AND USAGE** DUPIXENT is indicated for the following diseases: **1.1 Atopic Dermatitis** DUPIXENT is indicated for the treatment of adults and pediatric patients aged 6 months and older with moderate-to-severe atopic dermatitis who require chronic treatment and whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids. **1.2 Asthma** DUPIXENT is indicated in patients 6 years and older as an add-on maintenance treatment for severe asthma with type 2 inflammation characterized by elevated blood eosinophils and/or elevated FeNO. DUPIXENT is indicated as maintenance therapy for oral corticosteroid-dependent asthma. **1.3 Chronic Rhinosinusitis with Nasal Polyposis** DUPIXENT is indicated as an add-on maintenance treatment in adult patients with inadequately controlled severe chronic rhinosinusitis with nasal polyposis (CRSwNP). **1.4 Prurigo Nodularis** DUPIXENT is indicated for the treatment of adult patients with moderate-to-severe prurigo nodularis (PN) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids.
**4 CONTRAINDICATIONS** DUPIXENT is contraindicated in patients who have known hypersensitivity to dupilumab or any excipients of DUPIXENT _\[see Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
D11AH05
dupilumab
Manufacturer Information
SANOFI-AVENTIS SINGAPORE PTE. LTD.
Sanofi Winthrop Industrie
