Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2\. DOSAGE AND ADMINISTRATION** **2.1. Testing Prior to ESBRIET Administration** Conduct liver function tests prior to initiating treatment with ESBRIET _\[see Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.2. Recommended Dosage** The recommended daily maintenance dosage of ESBRIET is 801 mg three times daily for a total of 2403 mg/day. Doses should be taken with food at the same time each day. Upon initiation of treatment, titrate to the full dosage of 2403 mg/day over a 14-day period as follows: 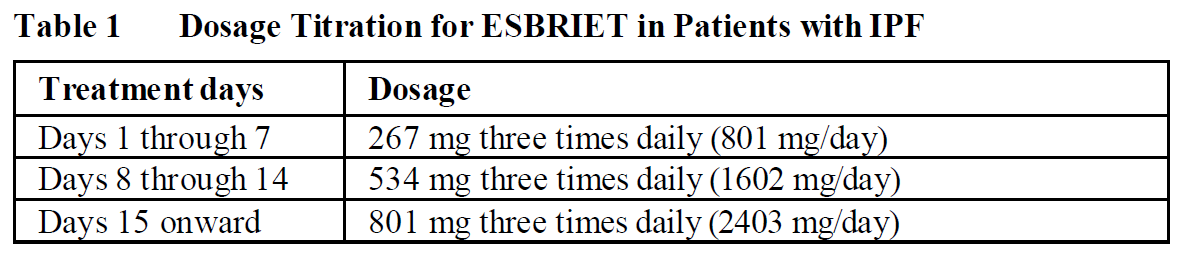 Dosages above 2403 mg/day are not recommended for any patient _\[see Overdosage (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.3. Dosage Modifications due to Adverse Reactions** Patients who miss 14 or more days of ESBRIET should re-initiate treatment by undergoing the initial 2-week titration regimen up to the full maintenance dosage _\[see Dosage and Administration (2.2)\]_. For treatment interruption of less than 14 days, the dosage prior to the interruption can be resumed. **Gastrointestinal events** In patients who experience intolerance to therapy due to gastrointestinal side effects, patients should be reminded to take the medicinal product with food. If symptoms persist, the dose of Esbriet may be reduced to 267 mg – 534 mg two to three times a day with food with re-escalation to the recommended daily dose as tolerated. If symptoms continue, patients may be instructed to interrupt treatment for one to two weeks to allow symptoms to resolve. **Photosensitivity reaction or rash** Patients who experience a mild to moderate photosensitivity reaction or rash should be reminded to use a sunblock daily and to avoid exposure to the sun (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The dose of Esbriet may be reduced to 801 mg each day (267 mg, three times daily). If the rash persists after 7 days, Esbriet should be discontinued for 15 days, with re-escalation to the recommended daily dose in the same manner as the dose escalation period. Patients who experience severe photosensitivity reaction or rash should be instructed to interrupt the dose and to seek medical advice (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Once the rash has resolved, Esbriet may be re-introduced and re-escalated up to the recommended daily dose at the discretion of the physician. Dosage Modification due to Elevated Liver Enzymes If a patient exhibits an aminotransferase elevation >3 to <5 × ULN without bilirubin elevation after starting ESBRIET therapy, other causes should be excluded, and the patient monitored closely. Discontinuation of other medicines associated with liver toxicity should be considered. If clinically appropriate, the dose of Esbriet should be reduced or interrupted. Once liver function tests are within normal limits Esbriet may be re-escalated to the recommended daily dose if tolerated. If a patient exhibits an aminotransferase elevation >3 to <5 × ULN accompanied by hyperbilirubinemia or clinical symptoms indicative of liver injury, Esbriet should be discontinued and the patient should not be rechallenged. If a patient exhibits an aminotransferase elevation to ≥5 × ULN, Esbriet should be discontinued and the patient should not be rechallenged. **2.4. Dosage Modification due to Drug Interactions** Strong CYP1A2 Inhibitors (e.g. fluvoxamine, enoxacin) Reduce ESBRIET to 267 mg three times a day (801 mg/day). Moderate CYP1A2 Inhibitors(e.g., ciprofloxacin) With use of ciprofloxacin at a dosage of 750 mg twice daily, reduce ESBRIET to 534 mg three times a day (1602 mg/day).
ORAL
Medical Information
**1\. INDICATIONS AND USAGE** ESBRIET is indicated for the treatment of idiopathic pulmonary fibrosis (IPF).
**4\. CONTRAINDICATIONS** None.
L04AX05
pirfenidone
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Delpharm Milano S.r.l.
Active Ingredients
Documents
Package Inserts
Esbriet PI.pdf
Approved: June 16, 2021
