Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Treatment should remain under the supervision of a physician who is experienced in the treatment of haematological diseases. Posology Romiplate® should be administered once weekly as a subcutaneous injection. _Initial dose_ _Chronic immune (idiopathic) thrombocytopenic purpura (ITP)_ The initial dose of romiplostim is 1 mcg/kg based on actual body weight. _Aplastic anaemia_ Usually administer an initial dose of 10 mcg/kg subcutaneously as romiplostim (genetical recombination) for adults. After initiation of treatment, the dose may be adjusted based on the patient’s condition, and administer once weekly. The maximum weekly dose is 20 mcg/kg. _Dose calculation_ The volume of romiplostim to administer is calculated based on body weight, dose required, and concentration of product. 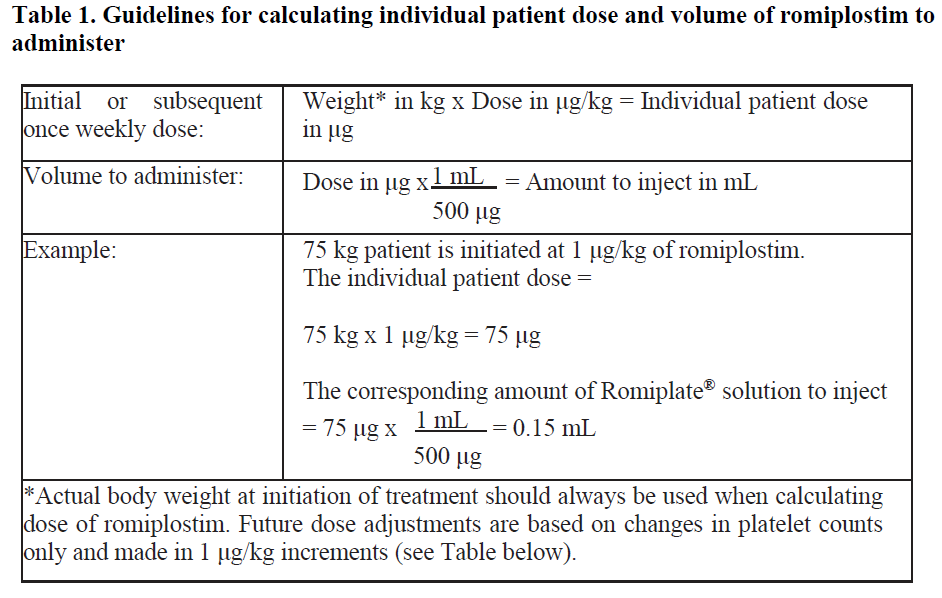 _Dose adjustments_ A subject’s actual body weight at initiation of therapy should be used to calculate dose. _Chronic immune (idiopathic) thrombocytopenic purpura (ITP)_ The once weekly dose of romiplostim should be increased by increments of 1 mcg/kg until the patient achieves a platelet count ≥ 50 x 109/L. Platelet counts should be assessed weekly until a stable platelet count (≥ 50 x 109/L for at least 4 weeks without dose adjustment) has been achieved. Platelet counts should be assessed monthly thereafter and appropriate dose adjustments made as per the dose adjustment table (table 2) in order to maintain platelet counts within the recommended range. See table 2 below for dose adjustment and monitoring. A maximum once weekly dose of 10 mcg/kg should not be exceeded. 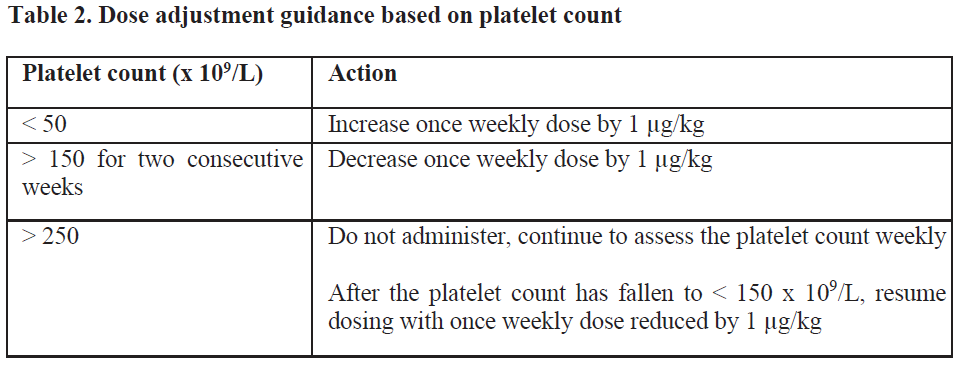 Due to the interindividual variable platelet response, in some patients platelet count may abruptly fall below 50 x 109/L after dose reduction or treatment discontinuation. In these cases, if clinically appropriate, higher cut-off levels of platelet count for dose reduction (200 x 109/L) and treatment interruption (400 x 109/L) may be considered according to medical judgement. A loss of response or failure to maintain a platelet response with romiplostim within the recommended dosing range should prompt a search for causative factors (see section 4.4, loss of response to romiplostim – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Aplastic anaemia_ Blood count should be measured weekly at the initial treatment phase and during the dose adjustment phase. Even if the dose has been maintained, it should be measured about once in 4 weeks. Generally, the dose should be adjusted with increments of 5 mcg/kg. Do not exceed a maximum once weekly dose of 20 mcg/kg. Dose increase should be considered in cases where platelet count has not risen (e.g. increase by ≥ 20 × 109/L from baseline or increase to ≥ 10 × 109/L and ≥ 100% increase from baseline with blood transfusion independence) though the same dose has been administered for 4 consecutive weeks. Use romiplostim at the lowest dose required for treatment in accordance with the following table: 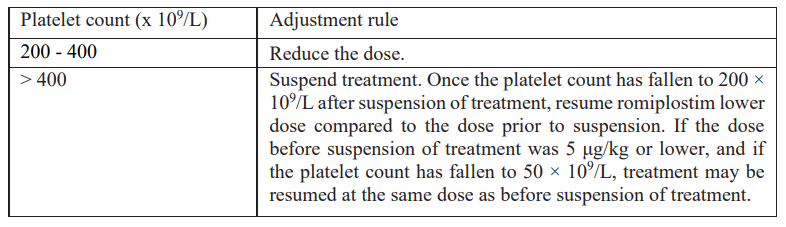 Reduce the dose in case where 3 blood cell lineage improvement is observed (e.g. platelet count above 50 × 109/L with blood transfusion independence, hemoglobin above 10 g/dL with blood transfusion independence, and neutrophil count above 1 × 109/L) for at least 8 consecutive weeks. If the improvement in 3 blood lineages has been maintained with the reduced dose for 4 weeks, further reduce the dose and consider subsequent dose reduction every 4 weeks (In the case of 5 mcg/kg or lower, consider suspension of treatment). If the condition has worsened in any of the 3 blood cell lineages, consider a dose increase (if the treatment has been suspended, it may be resumed at the previous dose). _Treatment discontinuation_ _Chronic immune (idiopathic) thrombocytopenic purpura (ITP)_ Treatment with romiplostim should be discontinued if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after four weeks of romiplostim therapy at the highest weekly dose of 10 mcg/kg. Patients should be clinically evaluated periodically and continuation of treatment should be decided on an individual basis by the treating physician, and in non-splenectomised patients this should include evaluation relative to splenectomy. The reoccurrence of thrombocytopenia is likely upon discontinuation of treatment (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Aplastic anaemia_ Appropriate measures such as discontinuing romiplostim should be taken in cases where none of the 3 blood lineages has improved even though the maximum weekly dose of 20 mcg/kg has been administered for 8 consecutive weeks. _Elderly patients (≥ 65 years)_ No overall differences in safety or efficacy have been observed in patients < 65 and ≥ 65 years of age (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Although based on these data no adjustment of the dosing regimen is required for older patients, care is advised considering the small number of elderly patients included in the clinical trials so far. _Paediatric population_ Romiplate® is not recommended for use in children below age 18 due to insufficient data on safety or efficacy. No recommendation on a posology can be made in this population. _Patients with hepatic impairment_ Romiplostim should not be used in patients with moderate to severe hepatic impairment (Child-Pugh score ≥ 7) unless the expected benefit outweighs the identified risk of portal venous thrombosis in patients with thrombocytopenia associated to hepatic insufficiency treated with thrombopoietin (TPO) agonists (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the use of romiplostim is deemed necessary, platelet count should be closely monitored to minimise the risk of thromboembolic complications. _Patients with renal impairment_ No formal clinical trials have been conducted in these patient populations. Romiplate® should be used with caution in these populations. Method of administration For subcutaneous use. After reconstitution of the powder, Romiplate® solution for injection is administered subcutaneously. The injection volume may be very small. Caution should be used during preparation of Romiplate® in calculating the dose and reconstitution with the correct volume of sterile water for injection. Special care should be taken to ensure that the appropriate volume of Romiplate® is withdrawn from the vial for subcutaneous administration – a syringe with graduations of 0.01 mL should be used. For instructions on reconstitution of Romiplate® before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Administration Precautions Caution should be used during preparation of Romiplate® in calculating the dose and reconstitution with the correct volume of sterile water for injection. Special care should be taken to ensure that the appropriate volume of Romiplate® is withdrawn from the vial for subcutaneous administration (see sections 4.4 for Special Warnings and Precautions for Use and 4.9 for Overdose – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** _Chronic immune (idiopathic) thrombocytopenic purpura (ITP)_ Romiplate® is indicated for adult chronic immune (idiopathic) thrombocytopenic purpura (ITP) splenectomised patients who are refractory to other treatments (e.g. corticosteroids, immunoglobulins) (see sections 4.2 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Romiplate® may be considered as second line treatment for adult non-splenectomised patients where surgery is contraindicated. _Aplastic anaemia_ Romiplate® is indicated for adults with moderate to severe aplastic anaemia refractory to conventional therapies.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 or to _E. coli_ derived proteins – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
B02BX04
romiplostim
Manufacturer Information
KYOWA KIRIN ASIA PACIFIC PTE. LTD.
Patheon Italia S.p.A Monza Operations
Kyowa Kirin Co., Ltd. Takasaki-Plant
Active Ingredients
Documents
Package Inserts
Romiplate Powder for Solution for Injection PI.pdf
Approved: April 14, 2023
