Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4 Dosage regimen and administration** Treatment with Piqray should be initiated by a physician experienced in the use of anticancer therapies. **Dosage regimen** **General target population** Patients with HR positive, HER2 negative advanced breast cancer should be selected for treatment with Piqray, based on the presence of a PIK3CA mutation in tumor or plasma specimens, using a validated test. If a mutation is not detected in a plasma specimen, test tumor tissue if available. There was no treatment benefit demonstrated in patients without PIK3CA mutations in the phase III clinical study (see section 12 clinical studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended dose of Piqray is 300 mg (2×150 mg film-coated tablets) taken orally, once daily on a continuous basis. Piqray should be taken immediately following food, at approximately the same time each day (see section 11 Clinical pharmacology and section 8 Interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The maximum recommended daily dose of Piqray is 300 mg. If a dose of Piqray is missed, it can be taken immediately following food and within 9 hours after the time it is usually administered. After more than 9 hours, the dose should be skipped for that day. On the next day, Piqray should be taken at its usual time. If the patient vomits after taking the Piqray dose, the patient should not take an additional dose on that day and should resume the usual dosing schedule the next day, at the usual time. When co-administered with Piqray, the recommended dose of fulvestrant is 500 mg administered intramuscularly on days 1, 15 and, 29, and once monthly thereafter. Please refer to the full prescribing information of fulvestrant. Treatment should continue as long as clinical benefit is observed or until unacceptable toxicity occurs. Dosing modifications may be necessary to improve tolerability. **Dosing modifications** The recommended daily dose of Piqray is 300 mg. Management of severe or intolerable adverse drug reactions (ADRs) may require temporary dosing interruption, reduction, and/or discontinuation of Piqray. If dosing reduction is required, the dosing reduction guidelines for ADRs are listed in Table 4-1. A maximum of 2 dosing reductions are recommended, after which the patient should be discontinued from treatment with Piqray. Dosing reduction should be based on worst preceding toxicity. 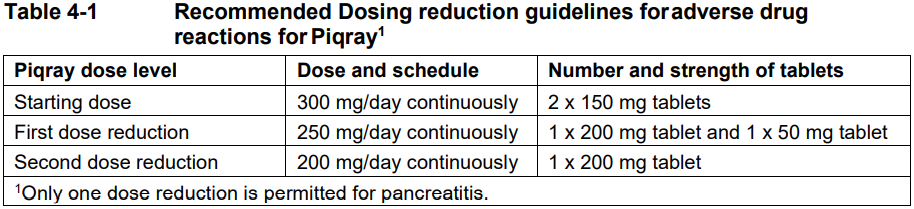 Tables 4-2, 4-3, 4-4 and 4-5 summarize recommendations for dosing interruption, reduction or discontinuation of Piqray in the management of specific ADRs. Clinical judgment of the treating physician, including confirmation of laboratory values if deemed necessary, should guide the management plan of each patient based on the individual benefit/risk assessment for treatment with Piqray. **Hyperglycaemia** Consultation with a Healthcare Professional (HCP) with experience in the management of hyperglycemia should be considered and lifestyle changes as per local guidelines (e.g. American Diabetes Association (ADA)), including exercise and dietary advice should be recommended/reinforced (e.g. small frequent meals, low carbohydrate, high fiber, low processed food intake, three macronutrient balanced meals and 2 optional small snacks rather than one large meal). In patients with risk factors for hyperglycemia, monitor fasting glucose more closely and as clinically indicated (see section 6 Warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 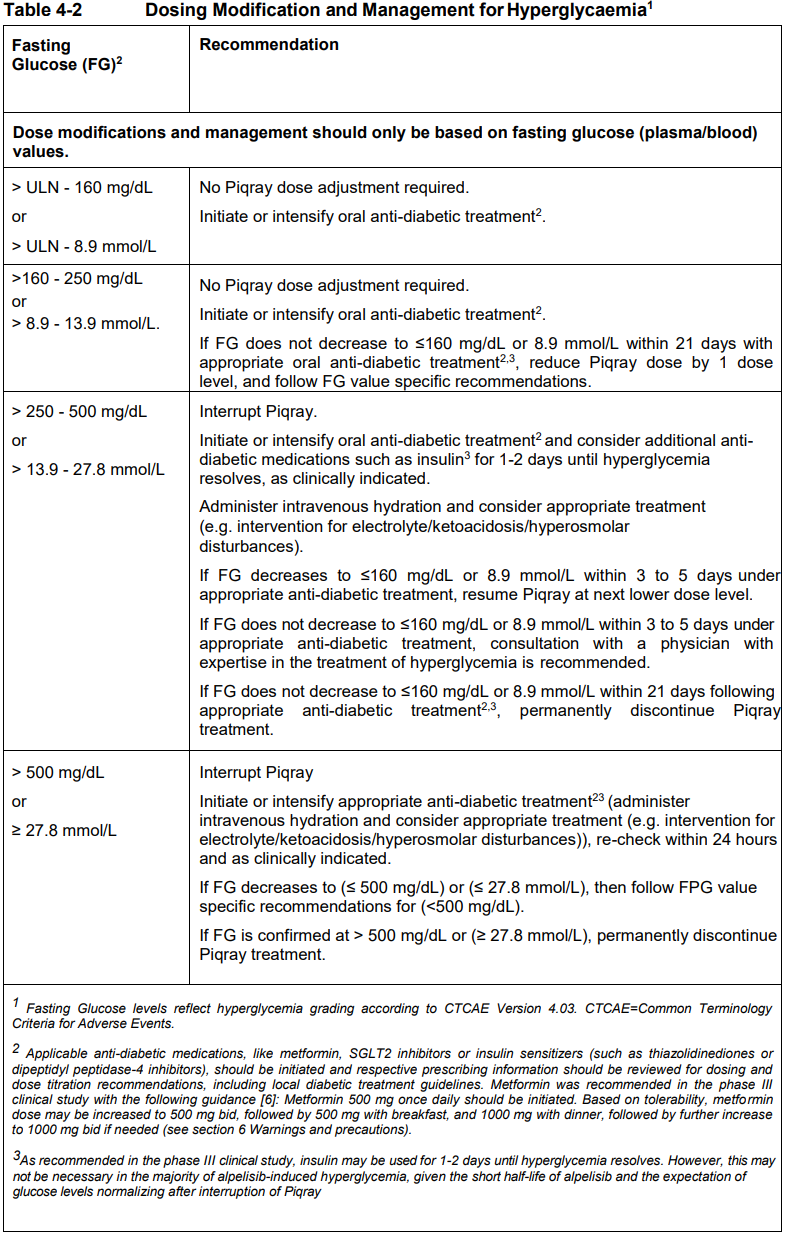 **Rash** Oral antihistamine administration may be considered prophylactically, at the time of initiation of treatment with Piqray. Based on the severity of rash, Piqray may require dose interruption, reduction, or discontinuation as described in Table 4-3 (see section 7 Adverse drug reactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 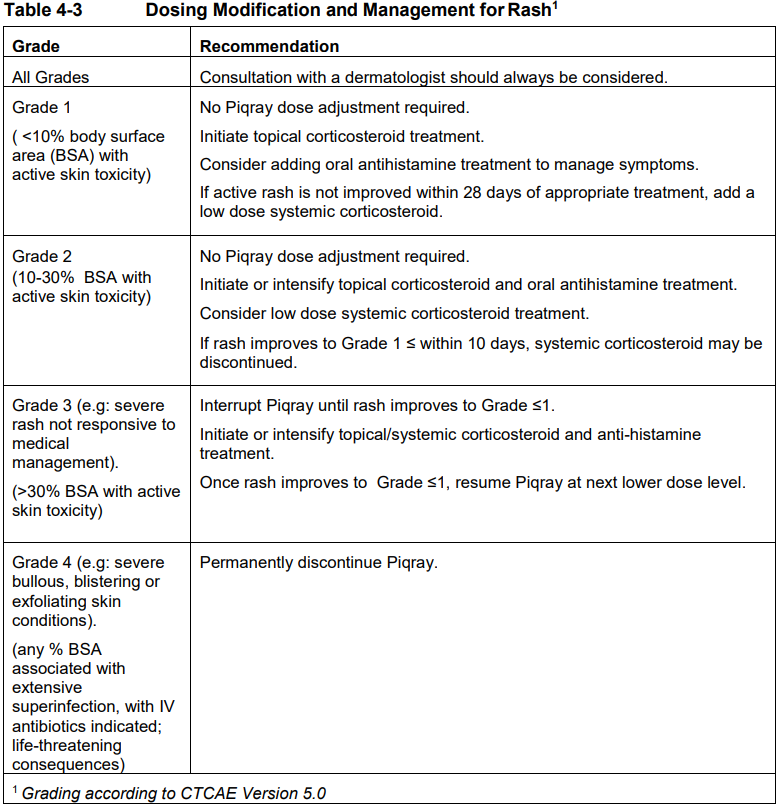 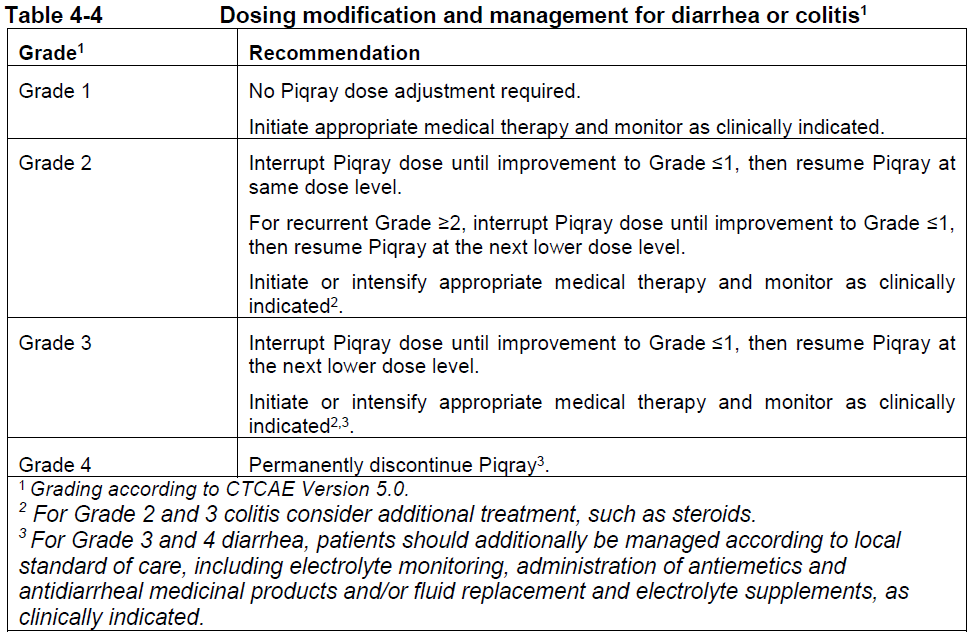 **Other toxicities** 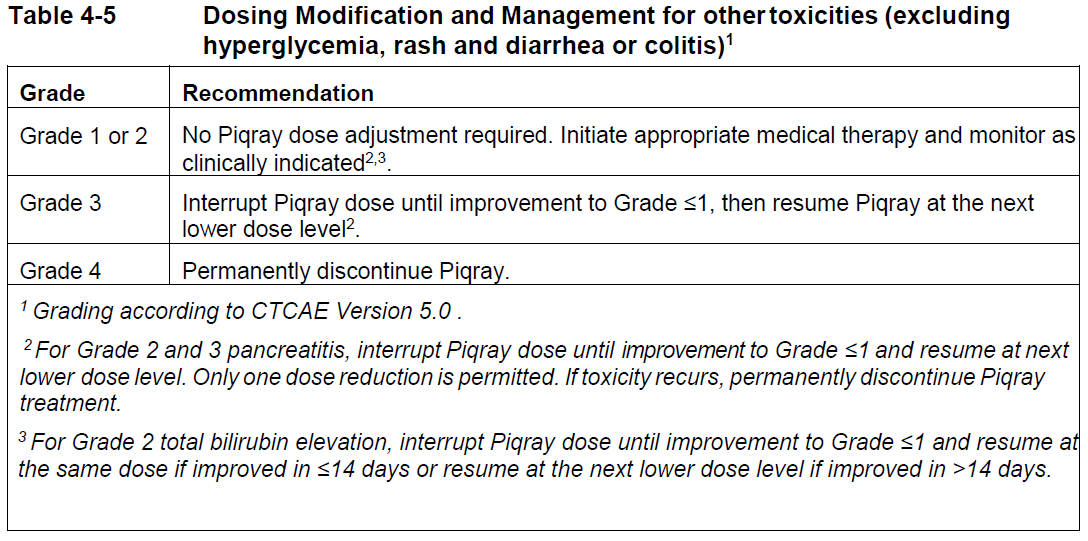 Refer to the full prescribing information for fulvestrant for dose modification guidelines in the event of toxicity and other relevant safety information. **Special populations** **Renal impairment** Based on population pharmacokinetic analysis, no dose adjustment is necessary in patients with mild or moderate renal impairment (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Caution should be used in patients with severe renal impairment as there is no experience with Piqray in this population (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** Based on a hepatic impairment study in non-cancer subjects with impaired hepatic function, no dose adjustment is necessary in patients with mild, moderate and severe hepatic impairment (Child-Pugh class A, B or C, respectively) (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Refer to the full prescribing information of fulvestrant for dose modifications related to hepatic impairment. **Pediatric patients (below 18 years)** The safety and efficacy of Piqray in pediatric patients have not been established. **Geriatric patients (65 years or above)** No dosage regimen adjustment is required in patients 65 years or above (see section 12 Clinical studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** Piqray tablets should be swallowed whole (tablets should not be chewed, crushed or split prior to swallowing). Tablets that are broken, cracked, or otherwise not intact should not be ingested.
ORAL
Medical Information
**3 Indications** Piqray® is an α-specific class I phosphatidylinositol-3-kinase (PIK3CA) inhibitor indicated for the treatment of postmenopausal women, and men, with hormone receptor positive, HER2- negative, advanced breast cancer with a PIK3CA mutation in combination with fulvestrant after disease progression following an endocrine-based regimen.
**5 Contraindications** Piqray is contraindicated in patients with hypersensitivity to the active substance or to any of the excipients.
Pending
xpending
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
NOVARTIS PHARMA STEIN AG
LEK PHARMACEUTICALS D.D. (Ljubljana) (Primary and Secondary packager)
LEK PHARMACEUTICALS D.D. (Lendava) (Primary and Secondary packager)
Active Ingredients
Documents
Package Inserts
Piqray daily dose pack PI.pdf
Approved: April 12, 2023
