Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and Administration** _TRANDATE_ injection is intended for i.v. use in hospitalised patients. Patients should always receive the drug whilst in the supine or left lateral position. Raising the patient into the upright position within 3 h of i.v. _TRANDATE_ administration should be avoided since excessive postural hypotension may occur. It is desirable to monitor the blood pressure and heart rate after injection and during infusion. In most patients, there is a small decrease in the heart rate; severe bradycardia is unusual but may be controlled by injecting atropine 1 to 2 mg intravenously. Respiratory function should be observed particularly in patients with any known impairment. _TRANDATE_ injection has been administered to patients with uncontrolled hypertension already receiving other hypotensive agents, including beta-blocking drugs, without adverse effects. **Populations** - **Adults** 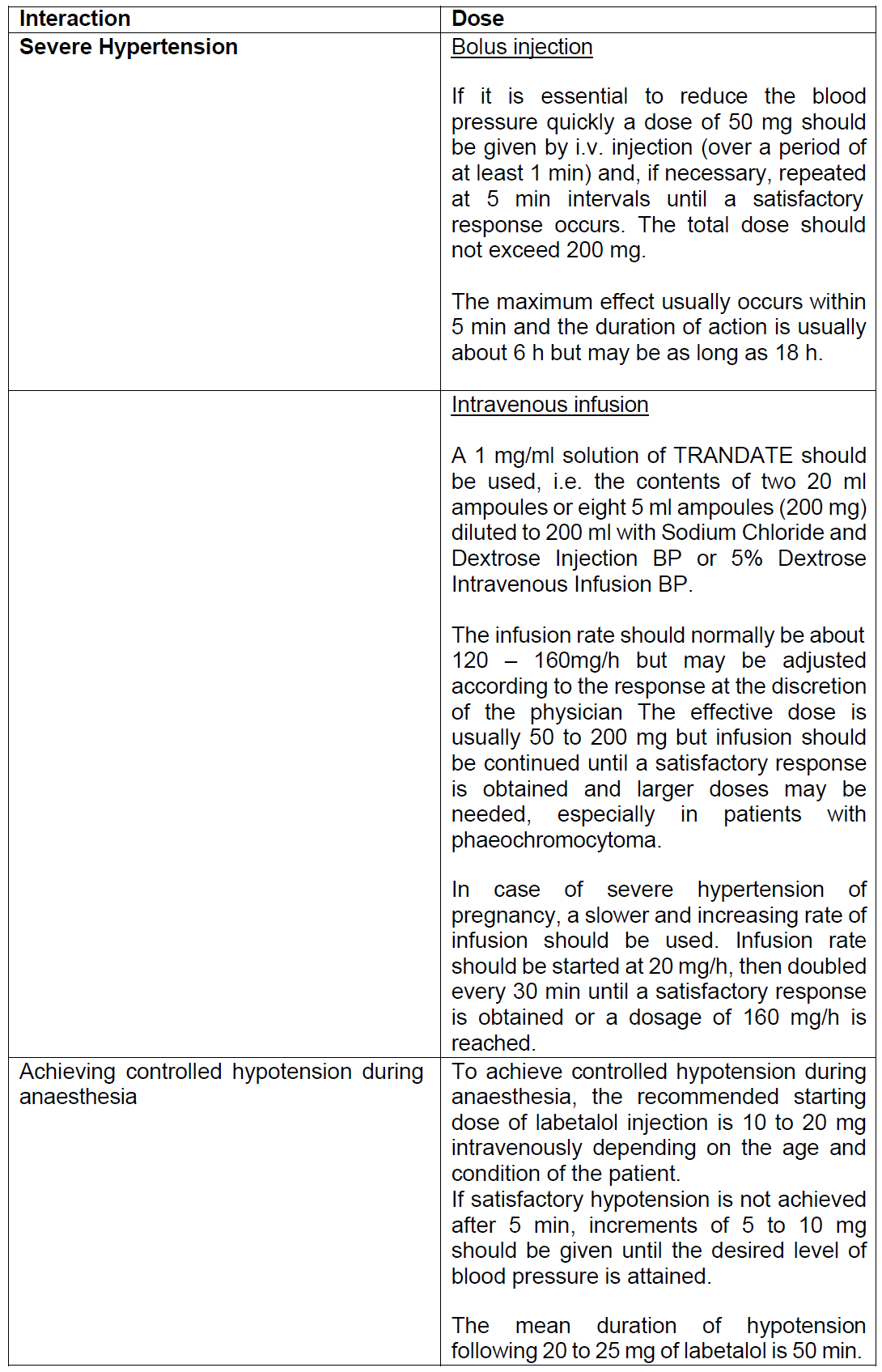 - **Paediatric population** Safety and efficacy in paediatric patients aged 0 to 18 years has not been established. No data are available.
INTRAVENOUS
Medical Information
**Indications** _TRANDATE injection is indicated for:_ - severe hypertension, including severe hypertension of pregnancy, when rapid control of blood pressure is essential - May be used to achieve control hypotension during anaesthesia
**Contraindications** _TRANDATE_ injection is contraindicated in second or third degree heart block (unless pacemaker is in situ), cardiogenic shock and other conditions associated with severe and prolonged hypotension or severe bradycardia. Non-selective beta-blockers should not be used in patients with asthma or a history of obstructive airway disease. Uncompensated heart failure Unstable/uncontrolled heart insufficiency Sick sinus syndrome (including sinus atrial block) unless pacemaker in situ Sinus node dysfunction Prinzmetal angina _TRANDATE_ injection is contraindicated for patients known to have hypersensitivity to the active substance or to any of the excipients listed.
C07AG01
labetalol
Manufacturer Information
DCH AURIGA SINGAPORE
UBI Pharma Inc
Active Ingredients
Documents
Package Inserts
Trandate Injection PI.pdf
Approved: April 14, 2023
