Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**2.2. DOSAGE AND ADMINISTRATION** **General** Substitution of Gazyva with any other biological medicinal product requires the consent of the prescribing physician. Gazyva should be administered as an intravenous infusion through a dedicated line in an environment where full resuscitation facilities are immediately available and under the close supervision of an experienced physician. Gazyva infusions should not be administered as an intravenous push or bolus. Isotonic 0.9% sodium chloride solution should be used as the infusion vehicle (see Section 4.2 Special Instructions for Use, Handling and Disposal – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Prophylaxis and Premedication for Tumour Lysis Syndrome (TLS)** Patients with a high tumour burden and/or a high circulating lymphocyte count (> 25 x 109/L) and/or renal impairment (CrCl <70 mL/min) are considered at risk of TLS and should receive prophylaxis. Prophylaxis should consist of adequate hydration and administration of uricostatics (e.g. allopurinol) or suitable alternative such as a urate oxidase (e.g. rasburicase), prior to start of Gazyva infusion as per standard practice (see Section 2.4.1 Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients should continue to receive repeated prophylaxis prior to each subsequent infusion, if deemed appropriate. **Prophylaxis and Premedication for Infusion Related Reactions (IRR)** Premedication to reduce the risk of infusion related reactions (see Section 2.4. Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) is outlined in Table 1. Corticosteroid premedication is recommended for FL patients and mandatory for CLL patients for the first infusion. Premedication for subsequent infusions and other premedication should be administered as described below. Hypotension, as a symptom of IRR, may occur during Gazyva intravenous infusions. Therefore, withholding of antihypertensive treatments should be considered for 12 hours prior to and throughout each Gazyva infusion and for the first hour after administration (see Section 2.4 Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 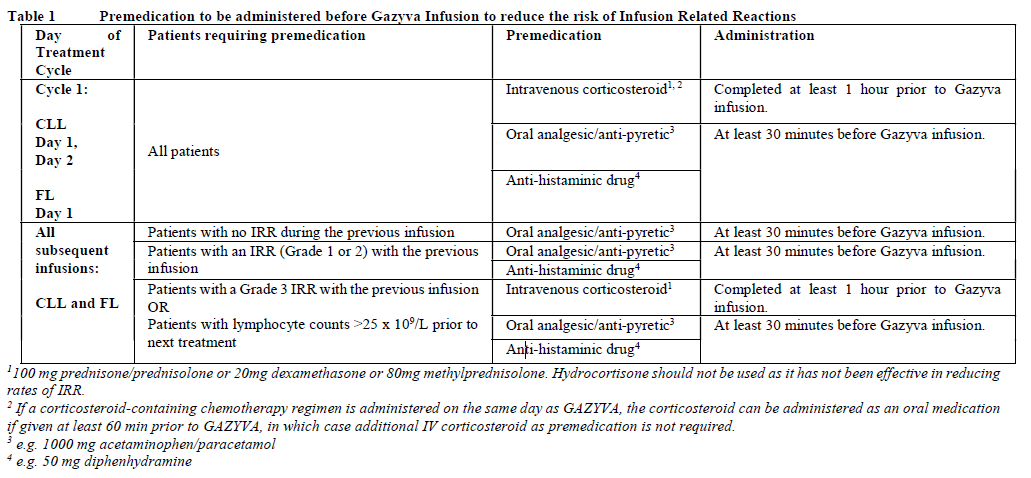 _**Standard Dosage**_ _**Chronic Lymphocytic Leukaemia (in combination with chlorambucil\*)**_ _Cycle 1_ The recommended dosage of Gazyva is 1000 mg administered over Day 1 and Day 2 and on Day 8 and Day 15 of the first 28 day treatment cycle as shown in Table 2. Two infusion bags should be prepared for the first dose, 100 mg for the first infusion and 900 mg for the second infusion. If the 100 mg dose is completed without modifications of the infusion rate or interruptions, the 900 mg dose can be administered on the same day (without dose delay) provided that appropriate time, conditions and medical supervision are available throughout the infusion. If there are any modifications of the infusion rate or interruptions during the first 100 mg the 900 mg infusion must be administered the following day (see Table 2). _Cycle 2–6_ The recommended dosage of Gazyva is 1000mg administered on Day 1 for each 28 day treatment cycle as shown in Table 2. 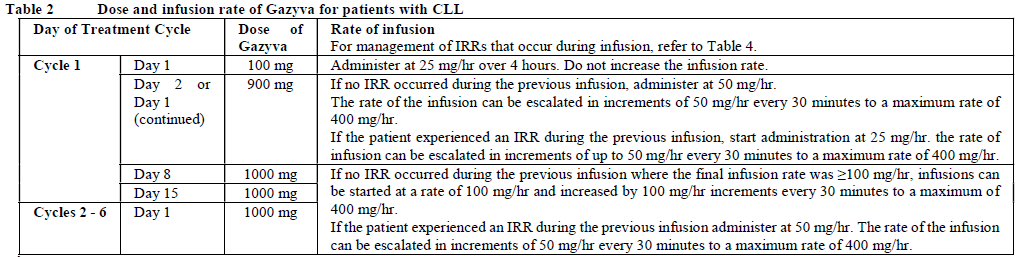 \*1 See section 3.1.2 Clinical/Efficacy Studies for information on chlorambucil dose – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _**Delayed or missed doses (CLL)**_ If a planned dose of Gazyva is missed, it should be administered as soon as possible; do not wait until the next planned dose. The planned treatment interval for Gazyva should be maintained between doses. _**Follicular Lymphoma**_ The recommended dosage of Gazyva is 1000 mg administered intravenously according to Table 3. _Previously Untreated Follicular Lymphoma_ For patients with previously untreated follicular lymphoma, GAZYVA should be administered with chemotherapy as follows: - Six 28 day cycles in combination with bendamustine2 or, - Six 21 day cycles in combination with CHOP, followed by 2 additional cycles of GAZYVA alone or, - Eight 21 day cycles in combination with CVP. Previously untreated patients who achieve a complete or partial response to GAZYVA plus chemotherapy should continue to receive GAZYVA (1000 mg) alone as maintenance therapy once every 2 months until disease progression or for up to 2 years. _Relapsed/Refractory Follicular Lymphoma_ For patients with follicular lymphoma who have relapsed after or who are refractory to rituximab or a rituximab-containing regimen, GAZYVA should be administered in six 28 day cycles in combination with bendamustine2. Relapsed/Refractory patients who achieve complete or partial response or have stable disease should continue to receive Gazyva 1000 mg alone as maintenance therapy once every 2 months until disease progression or for up to 2 years. GAZYVA should be administered at the standard infusion rate in Cycle 1 (see Table 3). In patients who do not experience Grade ≥3 infusion related reactions (IRRs) during Cycle 1, GAZYVA may be administered as a short (approximately 90 minutes) duration infusion (SDI) from Cycle 2 onwards (see Table 4). 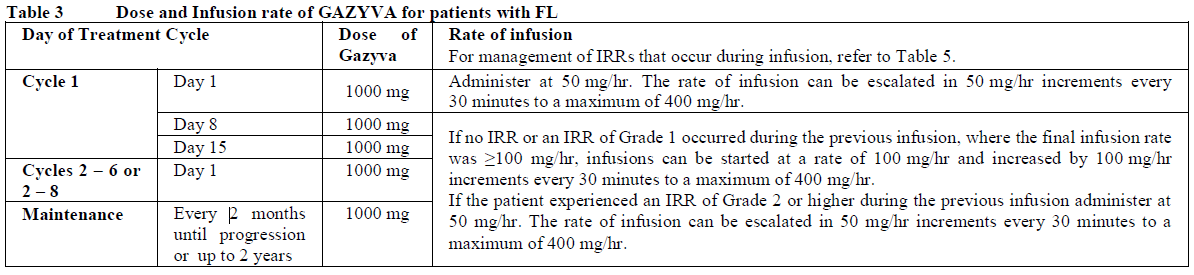 2 see section 3.1.2 Clinical/Efficacy Studies for information on bendamustine dose – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ 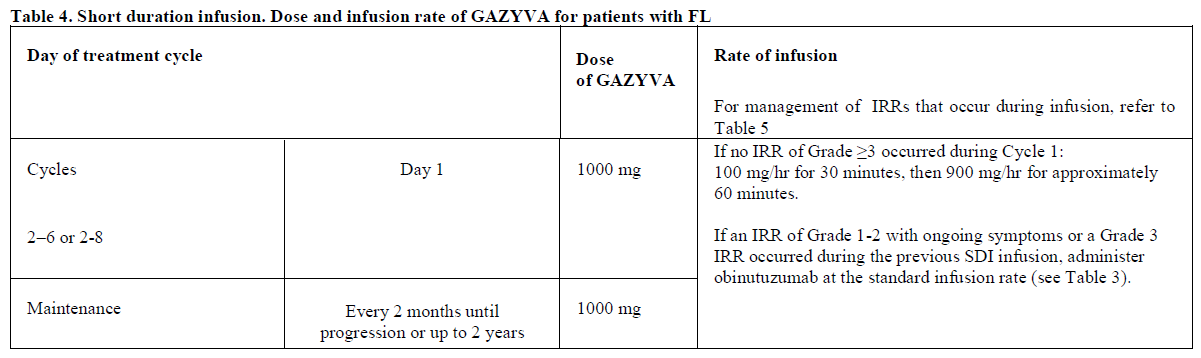 _**Delayed or missed doses (FL)**_ If a planned dose of Gazyva is missed, it should be administered as soon as possible; do not omit it or wait until the next planned dose. If toxicity occurs before Cycle 1 Day 8 or Cycle 1 Day 15, requiring delay of treatment, these doses should be given after resolution of toxicity. In such instances, all subsequent visits and the start of Cycle 2 will be shifted to accommodate for the delay in Cycle 1. During maintenance, maintain the original dosing schedule for subsequent doses. _**Dosage modifications during treatment (all indications)**_ No dose reductions of Gazyva are recommended. For management of symptomatic adverse events (including IRRs), see Table 4 below and section 2.4 Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. 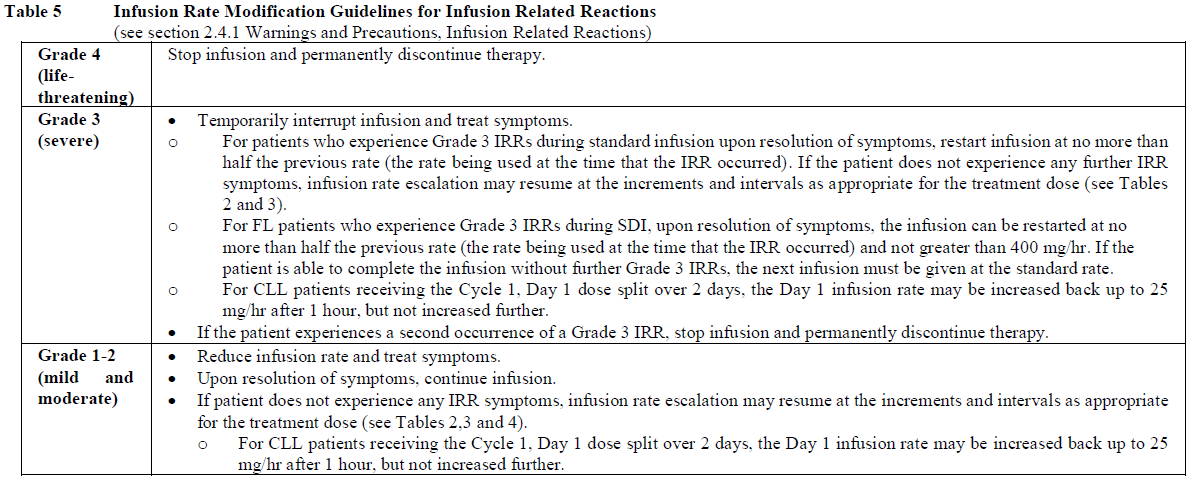 **2.2.1. SPECIAL DOSAGE INSTRUCTIONS** _Pediatric use_ The safety and efficacy of Gazyva in children below 18 years of age have not been established. _Geriatric use_ No dose adjustment is required in patients ≥ 65 years of age (see section 2.5.5 Use in Special Populations, Geriatric Use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No dose adjustment is required in patients with mild or moderate renal impairment. Gazyva has not been studied in patients with a CrCl ≤30mL/min (see section 2.5.5 Renal Impairment and 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic Impairment_ The safety and efficacy of Gazyva in patients with hepatic impairment have not been established.
INTRAVENOUS
Medical Information
**2.1. THERAPEUTIC INDICATION(S)** **Chronic Lymphocytic Leukaemia** Gazyva in combination with chlorambucil is indicated for the treatment of patients with previously untreated chronic lymphocytic leukaemia (CLL) see Section 3.1.2 Clinical/Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Follicular Lymphoma** GAZYVA in combination with chemotherapy, followed by GAZYVA maintenance in patients achieving a response, is indicated for the treatment of patients with previously untreated advanced follicular lymphoma. See Section 3.1.2 Clinical/Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Gazyva in combination with bendamustine, followed by Gazyva maintenance is indicated for the treatment of patients with follicular lymphoma (FL) who did not respond to, or who progressed during or up to 6 months after treatment with rituximab or a rituximab-containing regimen.
**2.3. CONTRAINDICATIONS** Gazyva is contraindicated in patients with a known hypersensitivity to obinutuzumab, murine proteins or to any of the excipients.
L01FA03
obinutuzumab
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Roche Diagnostics GmbH
Active Ingredients
Documents
Package Inserts
Gazyva PI.pdf
Approved: June 15, 2022
