Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Posology _Prevention of stroke and systemic embolism_ The recommended dose is 60 mg edoxaban once daily. Therapy with edoxaban in NVAF patients should be continued long term. _Treatment of DVT, treatment of PE and prevention of recurrent DVT and PE (VTE)_ The recommended dose is 60 mg edoxaban once daily following initial use of parenteral anticoagulant for at least 5 days (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Edoxaban and initial parenteral anticoagulant should not be administered simultaneously. The duration of therapy for treatment of DVT and PE (venous thromboembolism, VTE), and prevention of recurrent VTE should be individualised after careful assessment of the treatment benefit against the risk for bleeding (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Short duration of therapy (at least 3 months) should be based on transient risk factors (e.g. recent surgery, trauma, immobilisation) and longer durations should be based on permanent risk factors or idiopathic DVT or PE. For NVAF and VTE the recommended dose is 30 mg edoxaban once daily in patients with one or more of the following clinical factors: - Moderate or severe renal impairment (creatinine clearance (CrCL) 15 – 50 mL/min) - Low body weight ≤ 60 kg - Concomitant use of the following P-glycoprotein (P-gp) inhibitors: quinidine, ciclosporin, dronedarone, erythromycin, or ketoconazole. 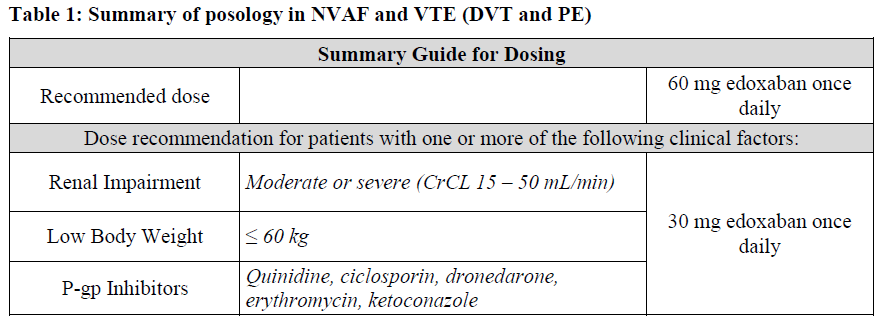 _Missed dose_ If a dose of Lixiana is missed, the dose should be taken immediately and then be continued the following day with the once-daily intake as recommended. The patient should not take double the prescribed dose on the same day to make up for a missed dose. _Switching to and from Lixiana_ Continued anticoagulant therapy is important in patients with NVAF and VTE. There may be situations that warrant a change in anticoagulation therapy (Table 2). 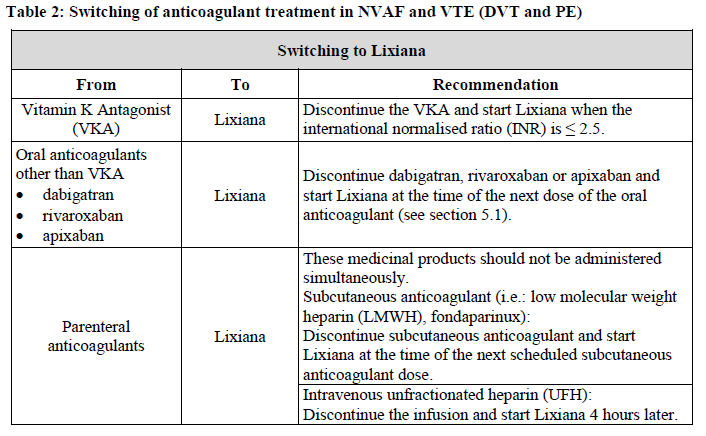 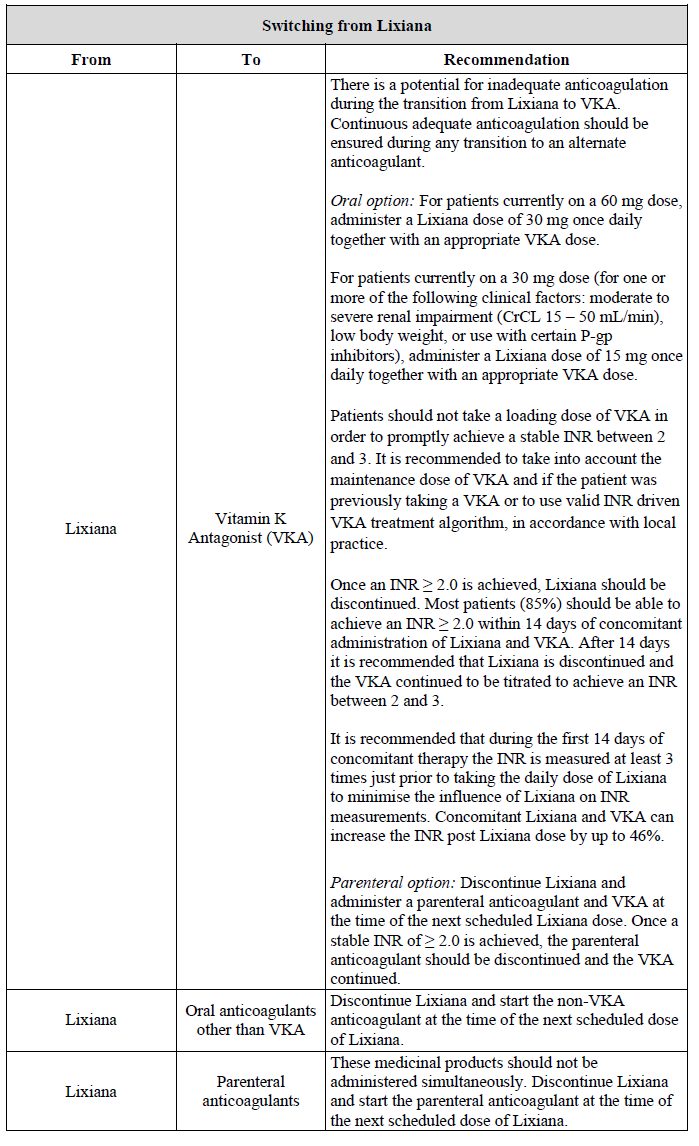 _Special populations_ _Elderly population_ No dose reduction is required (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ - Renal function should be assessed in all patients by calculating the creatinine clearance (CrCL) prior to initiation of treatment with Lixiana to exclude patients with end stage renal disease (i.e. CrCL < 15 mL/min), to use the correct Lixiana dose in patients with CrCL 15 – 50 mL/min (30 mg once daily), in patients with CrCL > 50 mL/min (60 mg once daily) and when deciding on the use of Lixiana in patients with increased creatinine clearance (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Renal function should also be assessed when a change in renal function is suspected during treatment (e.g. hypovolaemia, dehydration, and in case of concomitant use of certain medicinal products). The method used to estimate renal function (CrCL in mL/min) during the clinical development of Lixiana was the Cockcroft-Gault method. The formula is as follows: - For creatinine in micromole/L: 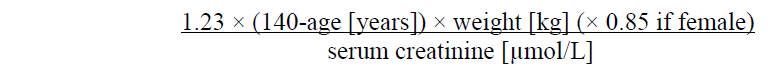 - For creatinine in mg/dL: 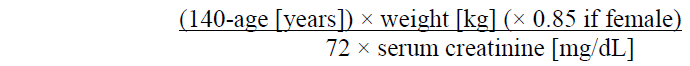 This method is recommended when assessing patients’ CrCL prior to and during Lixiana treatment. In patients with mild renal impairment (CrCL > 50 – 80 mL/min), the recommended dose is 60 mg Lixiana once daily. In patients with moderate or severe renal impairment (CrCL 15 – 50 mL/min), the recommended dose is 30 mg Lixiana once daily (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In patients with end-stage renal disease (ESRD) (CrCL < 15 mL/min) or on dialysis, the use of Lixiana is not recommended (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ Lixiana is contraindicated in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk (see section 4.3). In patients with severe hepatic impairment Lixiana is not recommended (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In patients with mild to moderate hepatic impairment the recommended dose is 60 mg Lixiana once daily (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Lixiana should be used with caution in patients with mild to moderate hepatic impairment (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with elevated liver enzymes (alanine aminotransferase (ALT) or aspartate transaminase (AST) > 2 x upper limit of normal (ULN)) or total bilirubin ≥ 1.5 x ULN, were excluded in clinical studies. Therefore Lixiana should be used with caution in this population (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Prior to initiating Lixiana, liver function testing should be performed. _Body weight_ For patients with body weight ≤ 60 kg, the recommended dose is 30 mg Lixiana once daily (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Gender_ No dose reduction is required (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Concomitant use of Lixiana with P-glycoprotein (P-gp) inhibitors_ In patients concomitantly taking Lixiana and the following P-gp inhibitors: quinidine, ciclosporin, dronedarone, erythromycin, or ketoconazole, the recommended dose is 30 mg Lixiana once daily (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No dose reduction is required for concomitant use of amiodarone or verapamil (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The use of Lixiana with other P-gp inhibitors including HIV protease inhibitors has not been studied. _Paediatric population_ The safety and efficacy of Lixiana in children and adolescents less than 18 years of age have not been established. No data are available. _Patients undergoing cardioversion_ Lixiana can be initiated or continued in patients who may require cardioversion. For transoesophageal echocardiogram (TEE) guided cardioversion in patients not previously treated with anticoagulants, Lixiana treatment should be started at least **2 hours** before cardioversion to ensure adequate anticoagulation (see sections 5.1 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Cardioversion should be performed no later than 12 hours after the dose of Lixiana on the day of the procedure. **For all patients undergoing cardioversion:** Confirmation should be sought prior to cardioversion that the patient has taken Lixiana as prescribed. Decisions on initiation and duration of treatment should follow established guidelines for anticoagulant treatment in patients undergoing cardioversion. Method of administration For oral use. Lixiana can be taken with or without food (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients who are unable to swallow whole tablets, Lixiana tablets may be crushed and mixed with water or apple puree and immediately administered orally (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Alternatively, Lixiana tablets may be crushed and suspended in a small amount of water and immediately delivered through a gastric tube after which it should be flushed with water (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Crushed Lixiana tablets are stable in water and apple puree for up to 4 hours.
ORAL
Medical Information
**4.1 Therapeutic indications** Lixiana is indicated in prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation (NVAF) with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack (TIA). Lixiana is indicated in treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and for the prevention of recurrent DVT and PE in adults (see section 4.4 for haemodynamically unstable PE patients – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. - Clinically significant active bleeding. - Hepatic disease associated with coagulopathy and clinically relevant bleeding risk. - Lesion or condition, if considered to be a significant risk for major bleeding. This may include current or recent gastrointestinal ulceration, presence of malignant neoplasms at high risk of bleeding, recent brain or spinal injury, recent brain, spinal or ophthalmic surgery, recent intracranial haemorrhage, known or suspected oesophageal varices, arteriovenous malformations, vascular aneurysms or major intraspinal or intracerebral vascular abnormalities. - Uncontrolled severe hypertension. - Concomitant treatment with any other anticoagulants e.g. unfractionated heparin (UFH), low molecular weight heparins (enoxaparin, dalteparin, etc.), heparin derivatives (fondaparinux, etc.), oral anticoagulants (warfarin, dabigatran etexilate, rivaroxaban, apixaban etc.) except under specific circumstances of switching oral anticoagulant therapy (see section 4.2) or when UFH is given at doses necessary to maintain an open central venous or arterial catheter (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Pregnancy and breast-feeding (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
B01AF03
edoxaban
Manufacturer Information
A. MENARINI SINGAPORE PTE. LTD.
Daiichi Sankyo Europe GmbH (Bulk manufacturer & primary packager)
Active Ingredients
Documents
Package Inserts
Lixiana PI.pdf
Approved: November 26, 2021
