Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**DOSAGE & ADMINISTRATION :** **Dosage & Dose Modifications :** Carboplatin should be used by intravenous route only. The recommended dose of carboplatin in previously untreated adults with normal renal function is 400mg/m2 given as a single short-term intravenous infusion over 15 to 60 minutes. Alternatively, see Formula dosing below. Therapy should not be repeated until 4 weeks after the previous carboplatin course and/or until the neutrophil count is at least 2,000 cells/mm3; and the platelet count is at least 100,000 cells/mm3. Initial dosage should be reduced by 20–25% in patients with risk factors such as previous myelosuppressive therapy and/or poor performance status. Determination of haematologic nadir by weekly blood counts during initial courses is recommended for future dosage adjustment and scheduling of carboplatin. **Impaired Renal Function** Patients with creatine clearance values below 60ml/min are at increased risk of severe myelosuppression. The frequency of severe leucopenia, neutropenia, of thrombocytopenia has been maintained at about 25% with the following dosage recommendations: 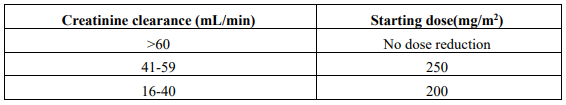 Insufficient data exists on the usage of carboplatin in patients with creatinine clearance of 15ml/min or less to permit a recommendation for treatment. All the above dosing recommendations apply to the initial course of treatment. Subsequent dosages should be adjusted according to the patient's tolerance and to the acceptable level of myelosuppression. **Combination Therapy** The optimal use of carboplatin in combination with other myelosuppressive agents require dosage adjustments according to the regimen and schedule to be adopted. **Formula Dosing** Another approach for determining the initial dose of carboplatin is the use of mathematical formulae, which are based on a patient's pre-existing renal function or renal function and desired platelet nadir. The use of dosing formulae, as compared to empiric dose calculation based on body surface area allows for adjustment for patient variations in pretreatment renal function that might otherwise result in underdosing in patients with above average renal function or overdosing in patients with impaired renal function. A simple formula for calculating dosage, based upon a patient's glomerular filtration rate (GFR in ml/min) and carboplatin target area under the concentration versus time curve (AUC in mg/ml\*min), as proposed by Calvert is: Dose (mg)= (target AUC) x (GFR + 25) **Note**: With the Calvert formula, the total dose of carboplatin is calculated in mg not mg/m2.  An approach in heavily pretreated patients\* receiving single agent carboplatin, when there is the desire to target a particular platelet nadir, is the use of the Egorin formula. Dose (mg/m2) = 0.091{Cr. Clearance (ml/min)/BSA (mg/m2} \[ {(Pretreatment platelet count-desired platelet nadir) x100/ Pretreatment platelet count}-17) + 86 \*Patients are considered heavily pretreated if they have received any of the following: mitomycin-C, nitrosoureas, combination therapy with doxorubicin, cyclophosphamide and cisplatin; chemotherapy with 5 or more different agents; or radiotherapy ≥ 4,500 rads to a single port 20x20 cm or more than one field of therapy.
INTRAVENOUS
Medical Information
**INDICATIONS:** Carboplatin is indicated in the treatment of: - Advanced ovarian cancer of epithelial origin - Small cell and non-small cell carcinoma of the lung - Squamous cell carcinoma of Head & neck - Advanced transitional cell carcinoma of the bladder (in combination with other agents) - Significant responses have been observed when carboplatin has been employed in the treatment of carcinoma of the cervix
**CONTRAINDICATIONS:** Carboplatin is contraindicated in patients with a history of severe allergic reactions to Cisplatin or other platinum containing compounds. Carboplatin should not be employed in patients with severe bone marrow depression or significant bleeding. Patients with pre-existing severe renal impairment.
L01XA02
carboplatin
Manufacturer Information
FRESENIUS KABI (SINGAPORE) PTE LTD
Fresenius Kabi Oncology Ltd.
Active Ingredients
Documents
Package Inserts
(DL7) PI_Kemocarb Injection_Baddi II_SGP_01_24 MAR 2021.pdf
Approved: May 20, 2021
