Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** INAPITANT is given for 3 days as part of a regimen that includes a corticosteroid and a 5-HT3 antagonist. The recommended dose of INAPITANT is 125 mg orally 1 hour prior to chemotherapy treatment (Day 1) and 80 mg orally once daily in the morning on Days 2 and 3. The package insert for the co-administered 5-HT3 antagonist must be consulted prior to initiation of treatment with INAPITANT. INAPITANT has not been studied for the treatment of established nausea and vomiting. Recommended dosing for the prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy: 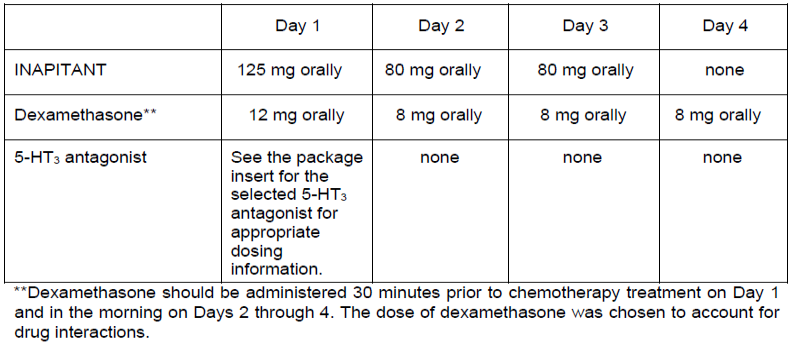 Recommended dosing for the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy: 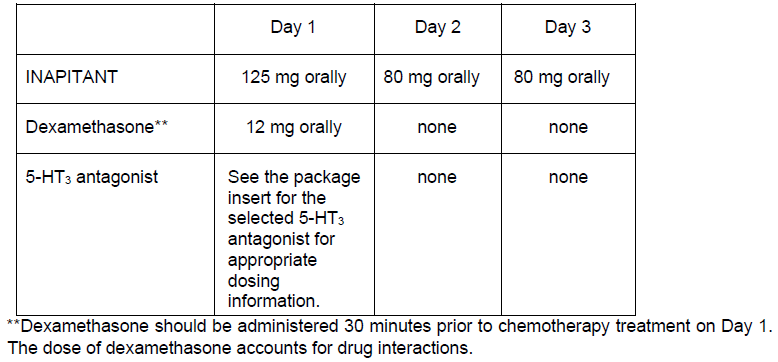 Chronic continuous administration is not recommended. See 4.5 for additional information on the administration of INAPITANT with corticosteroids – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Refer to the full prescribing information for co-administered antiemetic agents. INAPITANT may be taken with or without food. No dosage adjustment is necessary based on age, gender, race or Body Mass Index (BMI). No dosage adjustment is necessary for patients with severe renal insufficiency (creatinine clearance <30 mL/min) or for patients with end stage renal disease undergoing hemodialysis. No dosage adjustment is necessary for patients with mild to moderate hepatic insufficiency (Child-Pugh score 5 to 9). There are no clinical data in patients with severe hepatic insufficiency (Child-Pugh score >9).
ORAL
Medical Information
**4.1 Therapeutic indications** INAPITANT is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of: - Highly emetogenic cancer chemotherapy - Moderately emetogenic cancer chemotherapy INAPITANT should be given in combination with a corticosteroid and a 5-HT3 antagonist.
**4.3 Contraindications** Aprepitant is contraindicated in patients who are hypersensitive to any component of the product. Aprepitant should not be used concurrently with pimozide, terfenadine, astemizole, or cisapride. Inhibition of cytochrome P450 isoenzyme 3A4 (CYP3A4) by aprepitant could result in elevated plasma concentrations of these drugs, potentially causing serious or life-threatening reactions (see 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
A04AD12
aprepitant
Manufacturer Information
INTEGA PTE. LTD.
Pharmathen International SA
Active Ingredients
Documents
Package Inserts
INAPITANT TRI-PACK CAPSULE PI.pdf
Approved: November 5, 2021
