Regulatory Information
DCH AURIGA SINGAPORE
DCH AURIGA SINGAPORE
Therapeutic
Prescription Only
Formulation Information
ENTERIC COATED TABLET
**4.2 Posology and method of administration** Posology _Adults_ Depending upon the clinical requirements in individual cases, the following daily doses are recommended: 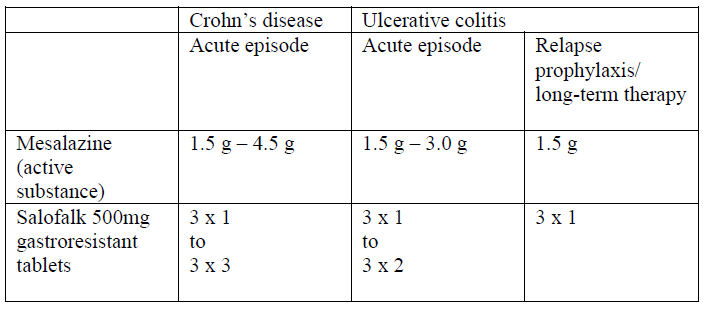 Method of administration Salofalk 500mg should be taken in the morning, at midday and in the evening, 1 hour before meals. They should be swallowed whole, not chewed, and taken with plenty of fluid. Treatment with Salofalk 500mg should be administered regularly and consistently, both during the acute inflammatory stage and during maintenance therapy in order to achieve the desired therapeutic effect. The duration of use is determined by the physician. An acute exacerbation of ulcerative colitis or Crohn’s disease generally subsides after 8–12 weeks. For relapse prophylaxis of ulcerative colitis the dose can usually be reduced to 1.5 g mesalazine/day. Note In rare cases, in patients who have undergone bowel resection/ bowel surgery in the ileocoecal region with removal of the ileocoecal valve, it has been observed that Salofalk 500mg, gastro-resistant tablets, were excreted undissolved in the stool, due to an excessively rapid intestinal passage.
ORAL
Medical Information
**4.1 Therapeutic indications** - Ulcerative colitis: treatment of acute exacerbations and relapse prophylaxis - Crohn’s disease: treatment of acute exacerbations
**4.3 Contraindications** Salofalk 500mg must not be administered to patients with - known hypersensitivity to the active substance, salicylates or any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information._ - severe impairment of hepatic or renal function.
A07EC02
mesalazine
Manufacturer Information
DCH AURIGA SINGAPORE
LOSAN PHARMA GMBH
ROTTENDORF PHARMA GMBH
ROTTENDORF PHARMA GMBH (Primary packager and Secondary packager)
LOSAN PHARMA GMBH (Primary packager and Secondary packager)
Active Ingredients
Documents
Package Inserts
Salofalk Tablet PI.pdf
Approved: July 29, 2022
