Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2\. DOSAGE AND ADMINISTRATION** Because of the rapid disintegration of the carbonate salt tablet and its rapid reaction with the hydrochloric acid in the stomach, the dosing of Renvela is anticipated to be similar to that of the hydrochloride salt. Renvela should be given three times a day with meals. _Patients Not Taking a Phosphate Binder._ The recommended starting dose of Renvela is 800 to 1600 mg, which can be administered as one or two Renvela 800 mg Tablets, with meals based on serum phosphorus level. Table 1 provides recommended starting doses of Renvela for patients not taking a phosphate binder. 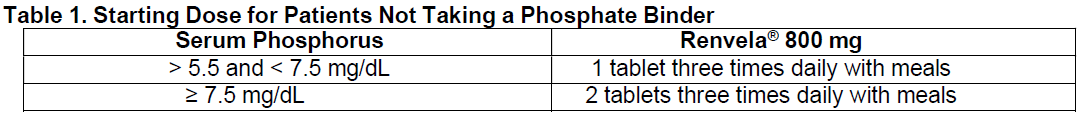 _Patients Switching from Sevelamer Hydrochloride._ For patients switching from sevelamer hydrochloride, sevelamer carbonate should be prescribed on a gram per gram basis. Further titration to the desired phosphate levels may be necessary. The highest daily dose of sevelamer carbonate studied was 14 grams in CKD patients on dialysis. _Patients Switching from Calcium Acetate._ In a study in 84 CKD patients on hemodialysis, a similar reduction in serum phosphorus was seen with equivalent doses (approximately mg for mg) of sevelamer hydrochloride and calcium acetate. Table 2 gives recommended starting doses of Renvela based on a patient’s current calcium acetate dose. 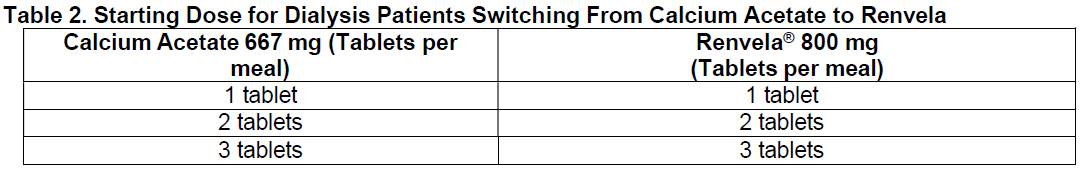 _Dose Titration for All Patients Taking Renvela._ The dose should be increased or decreased by one tablet per meal at two week intervals, as necessary, with the goal of controlling serum phosphorus within the target range of 3.5 mg/dL to 5.5 mg/dL.
ORAL
Medical Information
**1\. INDICATIONS AND USAGE** Renvela is indicated for the control of hyperphosphataemia in adult patients receiving hemodialysis or peritoneal dialysis. Renvela is also indicated for the control of hyperphosphataemia in adult patients with chronic kidney disease not on dialysis with serum phosphorus ≥ 1.78 mmol/l. Renvela should be used within the context of a multiple therapeutic approach, which could include calcium supplement, 1,25-dihydroxy Vitamin D3 or one of its analogues to control the development of renal bone disease.
**4\. CONTRAINDICATIONS** Renvela is contraindicated in patients with hypophosphatemia or bowel obstruction. Renvela is contraindicated in patients with hypersensitivity to sevelamer carbonate, sevelamer hydrochloride, or to any of the excipients.
V03AE02
sevelamer
Manufacturer Information
SANOFI-AVENTIS SINGAPORE PTE. LTD.
Sanofi Winthrop Industrie
Active Ingredients
Documents
Package Inserts
Renvela Tablet PI.pdf
Approved: June 23, 2023
