Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, CHEWABLE
**HOW TO TAKE ZENTEL** The recommended dose of **ZENTEL** is as follows: Adults and children over 2 years of age 400 mg as a single dose. Children 1–2 years of age 200 mg as a single dose. **ZENTEL** should not to be used in children aged under 1 year. No special procedures, such as fasting or purging, are required. If the patient is still symptomatic after a single course of treatment, they must consult a Healthcare Professional for further treatment. The maximum duration of treatment will vary according to your condition. Please refer to the table below for information on maximum doses for each condition. Do not exceed the maximum daily doses and treatment durations recommended. Some people, particularly young children, may experience difficulties swallowing the tablets whole and should be encouraged to chew the tablets with a little water; alternatively the tablets may be crushed. The suspension can also be administered as an alternative. 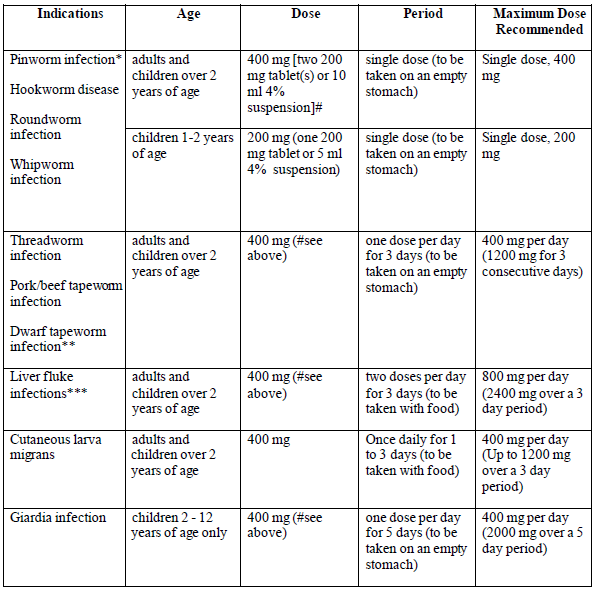 \*In order to obtain a complete cure in the case of pin-worm infestation, measures should be taken to maintain strict hygiene. Relatives and individuals sharing the same housing should also take a course of treatment. \*\*In cases of proven dwarf tapeworm infection (Hymenolepiasis), a repeat course of the medicine in 10–21 days is recommended. \*\*\*Advice should be sought from your Healthcare Professional 1 month after treatment to confirm fluke eradication. - Elderly Elderly patients with hepatic impairment (liver problems) must seek advice from a Healthcare Professional before taking this medicine. - Renal Impairment (Kidney problems) If you have been diagnosed with renal impairment (kidney problems), seek advice from a Healthcare Professional before taking this medicine. - Hepatic Impairment (Liver problems) If you have been diagnosed with hepatic impairment (liver problems), seek advice from a Healthcare Professional before taking this medicine.
ORAL
Medical Information
**WHAT ZENTEL IS AND WHAT IT IS USED FOR** **ZENTEL** is a benzimidazole carbamate with anthelmintic and antiprotozoal activity against the intestinal and tissue worm infections. **ZENTEL** is used for the treatment of common worm infections of the gut, such as the following conditions caused by sensitive intestinal helminths/protozoa: - Pinworm infection (enterobiasis) - Hookworm disease (ancylostomiasis and necatoriasis) - Dwarf tapeworm infection (hymenolepsiasis) - Pork/beef tapeworm infections (taeniasis) - Threadworm infection (strongyloidiasis) - Roundworm infection (ascariasis) - Whipworm infection (trichuriasis) - Liver fluke infections (clonorchiasis and opisthorchiasis) - Hookworm (animal origin) causing skin disease (cutaneous larva migrans) - Giardia infection (giardiasis in children)
**DON’T TAKE ZENTEL** If you are pregnant, if you think you could be pregnant, or if you are planning to become pregnant (see PREGNANCY AND BREAST FEEDING – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If you are allergic (hypersensitive) to **ZENTEL** or any other ingredients of **ZENTEL** (see section on WHAT **ZENTEL** CONTAINS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
P02CA03
albendazole
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
GlaxoSmithKline Consumer Healthcare South Africa (Pty) Limited
Active Ingredients
Documents
Patient Information Leaflets
Zentel PIL.pdf
Approved: March 22, 2021
