Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**2.2 DOSAGE AND ADMINISTRATION** **Patient Selection** Patients treated with Phesgo should have HER2-positive tumor status, defined as a score of 3+ by immunohistochemistry (IHC) and/or a ratio of ≥ 2.0 by in situ hybridization (ISH), assessed by a validated test. To ensure accurate and reproducible results, the testing must be performed in a specialized laboratory, which can ensure validation of the testing procedures. For full instructions on assay performance and interpretation, please refer to the package inserts of validated HER2 testing assays. **Administration of Phesgo** Phesgo therapy should only be administered under the supervision of a healthcare professional experienced in the treatment of cancer patients. Substitution by any other biological medicinal product requires the consent of the prescribing physician. Patients currently receiving intravenous Perjeta and Herceptin can switch to Phesgo. Switching treatment from intravenous Perjeta and Herceptin to Phesgo (or vice versa) was investigated in study MO40628 ( _see section 2.6.1 Undesirable Effects and section 3.1.2 Clinical / Efficacy Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In order to prevent medication errors, it is important to check the vial labels to ensure that the drug being prepared and administered is Phesgo. Phesgo is for subcutaneous (SC) use in the thigh only. Do not administer intravenously. **Metastatic and Early Breast Cancer** For Phesgo dose recommendations in early and metastatic breast cancer refer to Table 1. 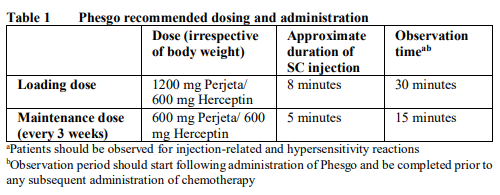 In patients receiving intravenous Perjeta and Herceptin with < 6 weeks since their last dose, Phesgo should be administered as a maintenance dose of 600 mg Perjeta/600 mg Herceptin and every 3 weeks for subsequent administrations. In patients receiving intravenous Perjeta and Herceptin with ≥ 6 weeks since their last dose, Phesgo should be administered as a loading dose of 1200 mg Perjeta/600 mg Herceptin, followed by a maintenance dose of 600 mg Perjeta/600 mg Herceptin every 3 weeks for subsequent administrations. The injection site should be alternated between the left and right thigh only. New injections should be given at least 1 inch/2.5 cm from the previous site on healthy skin and never into areas where the skin is red, bruised, tender, or hard. Do not split the dose between two syringes or between two sites of administration. During the treatment course with Phesgo, other medications for SC administration should preferably be injected at different sites. In patients receiving a taxane, Phesgo should be administered prior to the taxane. When administered with Phesgo, the recommended initial dose of docetaxel is 75 mg/m2, and the dose of docetaxel may be escalated to 100 mg/m2 on subsequent cycles if the initial dose is well tolerated. In patients receiving an anthracycline-based regimen, Phesgo should be administered following completion of the entire anthracycline regimen. **Early Breast Cancer (EBC)** In the neoadjuvant setting (before surgery), it is recommended that patients are treated with Phesgo for three to six cycles depending on the regimen chosen in combination with chemotherapy ( _see 3.1.2 Clinical/ Efficacy Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In the adjuvant setting (after surgery), Phesgo should be administered for a total of one year (up to 18 cycles or until disease recurrence, or unmanageable toxicity, whichever occurs first), as part of a complete regimen for early breast cancer and regardless of the timing of surgery. Treatment should include standard anthracycline and/or taxane-based chemotherapy. Phesgo treatment should start on Day 1 of the first taxane-containing cycle and should continue even if chemotherapy is discontinued ( _see 3.1.2 Clinical/Efficacy Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Metastatic Breast Cancer (MBC)** Phesgo should be administered in combination with docetaxel until disease progression or unmanageable toxicity. Treatment with Phesgo may continue even if treatment with docetaxel is discontinued. **Delayed or Missed Doses** If the time between two sequential doses is less than 6 weeks, the 600 mg Perjeta/ 600 mg Herceptin maintenance dose of Phesgo should be administered as soon as possible. Do not wait until the next planned dose. If the time between two sequential injections is 6 weeks or more, the loading dose of 1200 mg Perjeta/600 mg Herceptin should be re-administered followed by the maintenance dose of 600 mg Perjeta/ 600 mg Herceptin every 3 weeks thereafter. **Dose Modifications** No dose reductions of Phesgo are recommended. For chemotherapy dose modifications, see relevant prescribing information. _Injection-related reactions_ The injection should be slowed or paused if the patient experiences injection-related symptoms ( _see 2.4 Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hypersensitivity/anaphylaxis_ The injection should be discontinued immediately and permanently if the patient experiences a serious hypersensitivity reaction (e.g. anaphylaxis) ( _see 2.4 Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Left ventricular dysfunction_ Assess LVEF prior to initiation of Phesgo and at regular intervals during treatment to ensure that LVEF is within normal limits (see Table 2 below). If the LVEF declines as indicated in Table 2 and has not improved, or has declined further at the subsequent assessment, discontinuation of Phesgo should be strongly considered, unless the benefits for the individual patient are deemed to outweigh the risks. See _2.4 Warnings and Precautions_ for more information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.  **2.2.1 Special Dosage Instructions** **Pediatric use** The safety and efficacy of Phesgo in children and adolescents (< 18 years) has not been established. **Geriatric use** No dose adjustment of Phesgo is required in patients ≥ 65 years of age ( _see 2.5.5 Geriatric Use and 3.2.5 Pharmacokinetics in Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal Impairment** Dose adjustments of Phesgo are not needed in patients with mild or moderate renal impairment. No dose recommendations can be made for patients with severe renal impairment because of the limited pharmacokinetic data available ( _see 3.2.5 Pharmacokinetics in special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic Impairment** The safety and efficacy of Phesgo have not been studied in patients with hepatic impairment. No dose recommendation can be made for Phesgo ( _see 3.2.5 Pharmacokinetics in Special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
SUBCUTANEOUS
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** **Early Breast Cancer (EBC)** Phesgo is indicated for use in combination with chemotherapy for the: - neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer ( _see 3.1.2 Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). This indication is based on demonstration of an improvement in pathological complete response rate. No data are available demonstrating improvement in event-free survival or overall survival. - adjuvant treatment of patients with HER2-positive early breast cancer at high risk of recurrence _(see 3.1.2 Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) **Metastatic Breast Cancer (MBC)** Phesgo is indicated for use in combination with docetaxel for the treatment of adult patients with HER2-positive metastatic or locally recurrent unresectable breast cancer, who have not received previous anti-HER2 therapy or chemotherapy for their metastatic disease.
**2.3 CONTRAINDICATIONS** Phesgo is contraindicated in patients with a known hypersensitivity to Perjeta, Herceptin, or any of the excipients.
L01XY02
xl 01 xy 02
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
F. Hoffmann-La Roche Ltd
Active Ingredients
Documents
Package Inserts
Phesgo PI.pdf
Approved: June 22, 2021
