Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**4.2 Posology and method of administration** Treatment should be initiated and administered under medical supervision, e.g. in the hospital setting. Home based treatment can be recommended in exceptional cases (severe symptoms or experienced patients requiring higher doses) when a low dose rapid acting beta-agonist bronchodilator such as BERODUAL® metered dose inhaler has been insufficient in providing relief after consultation with an experienced physician. It can also be recommended in patients who are in need for nebuliser treatment for other reasons e.g. handling issues of MDI or requirement of higher doses in experienced patients. The treatment with the nebuliser solution in UDVs should always be started with the lowest recommended dose (1 UDV). In very severe cases, two unit dose vials may be required for symptom relief. The dosage should be adapted to the individual requirements and tailored according to the severity of the acute episode. Administration should be stopped when sufficient symptom relief is achieved. The following dosages are recommended for adults (including elderly patients) and adolescents > 12 years: **Acute episodes of bronchospasm** 1 unit dose vial is sufficient for prompt symptom relief in most cases, typically the hospital-based treatment of moderate to severe asthma attacks or the home- and hospital-based treatment of patients with moderate to severe COPD. In very severe cases, two unit dose vials may be required for symptom relief. These should be administered under medical supervision. Children ≤ 12 years: Because of insufficient information the general use in children ≤12 years of age is not recommended. **Instructions for use** This solution is ready for use and requires no dilution. The unit dose vials are intended only for inhalation with suitable nebulising devices and must not be taken orally or administered parenterally. DUOVENT® nebuliser solution can be administered using a range of commercially available nebulising devices. The lung and systemic drug exposure is dependent on the nebuliser used and may be higher than with BERODUAL® metered dose inhaler depending on the efficiency of the device. Where wall oxygen is available the solution is best administered at a flow rate of 6 – 8 litres per minute. The instructions provided by the manufacturer of the nebulising device for proper care, maintenance and cleaning of the equipment should be followed. 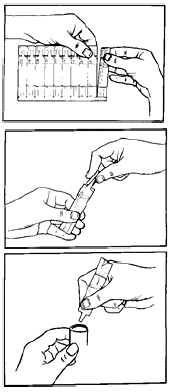 1. Prepare the nebuliser for filling, according to the instructions provided by the manufacturer or physician. 2. Tear one unit dose vial from the strip. 3. Open the unit dose vial by firmly twisting the top. 4. Squeeze the content of the unit dose vial into the nebuliser reservoir. 5. Assemble the nebuliser and use as directed. 6. After use throw away any solution left in the reservoir and clean the nebuliser, following the manufacturer's instructions. Since the unit dose vials contain no preservative, it is important that the contents are used soon after opening and that a fresh vial is used for each administration to avoid microbial contamination. Partly used, opened or damaged unit dose vials should be discarded.
NASAL
Medical Information
**4.1 Therapeutic Indications** DUOVENT® is a bronchodilator for the prevention and treatment of symptoms in chronic obstructive airway disorders with reversible airflow limitation such as bronchial asthma and especially chronic bronchitis with or without emphysema. Concomitant anti-inflammatory therapy should be considered for patients with bronchial asthma and steroid responsive chronic obstructive pulmonary disease (COPD).
**4.3 Contraindications** DUOVENT® is contraindicated in patients with known hypersensitivity to fenoterol hydrobromide or atropine-like substances or to any of the excipients of the product. DUOVENT® is also contraindicated in patients with hypertrophic obstructive cardiomyopathy and tachyarrhythmia.
R03AK03
xr 03 ak 03
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
Laboratoire Unither
Active Ingredients
Ipratropium bromide monohydrate 0.52mg/4ml eqv. to Ipratropium bromide anhydrous
0.5 mg/4 ml
Documents
Package Inserts
Duovent UDVS Nebuliser Solution PI.pdf
Approved: April 21, 2023
