Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2.2 Dosage and Administration** _General_ Treatment with Zelboraf should be initiated and supervised by a qualified physician experienced in the use of anticancer medicinal products. Patients treated with Zelboraf must have a previously confirmed BRAF V600 mutation-positive tumor status by a validated test. (see section 2.4 and 3.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) _Standard Dosage_ The recommended dose of Zelboraf is 960 mg (four 240 mg tablets) twice daily. Zelboraf may be taken with or without food, but consistent intake of both daily doses on an empty stomach should be avoided (see Section 3.2.1 Absorption – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Zelboraf tablets should be swallowed whole with a glass of water. Zelboraf tablets should not be chewed or crushed. _Duration of Treatment_ It is recommended that treatment with Zelboraf continue until disease progression or the development of unacceptable toxicity (see Tables 1 and 2). _Missed Doses_ If a planned dose is missed, it can be taken up to 4 hours prior to the next dose to maintain the twice-daily regimen. Both doses should not be taken at the same time. _Vomiting_ In case of vomiting after Zelboraf administration the patient should not take an additional dose of the medicinal product but the treatment should be continued as usual. _Dose Modifications (see sections 2.4.1 General, Warnings and Precautions and section 2.6.1 Clinical Trials, Undesirable Effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_ Management of symptomatic adverse events or prolongation of QTc may require dose reduction, temporary interruption or treatment discontinuation of Zelboraf. Dose modifications or interruptions are not recommended for cutaneous squamous cell carcinoma (cuSCC). Dose reductions resulting in a dose below 480 mg twice daily are not recommended. 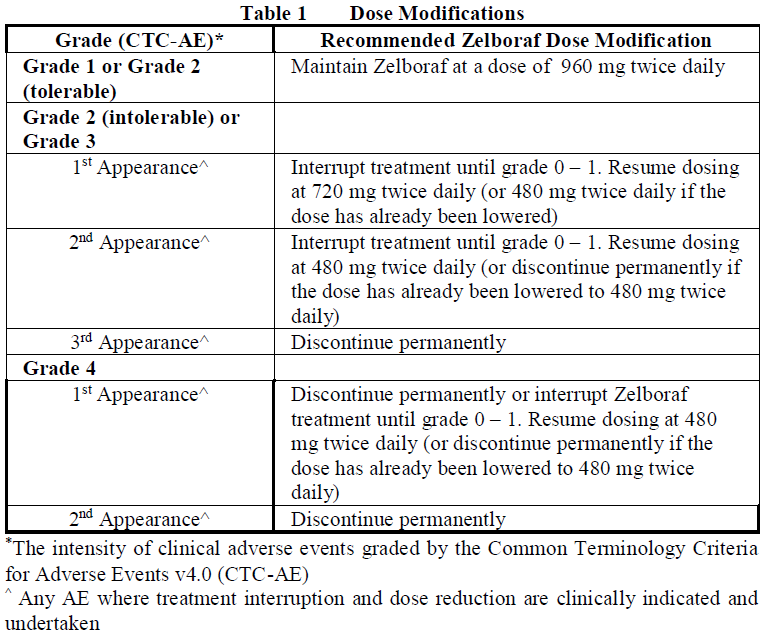 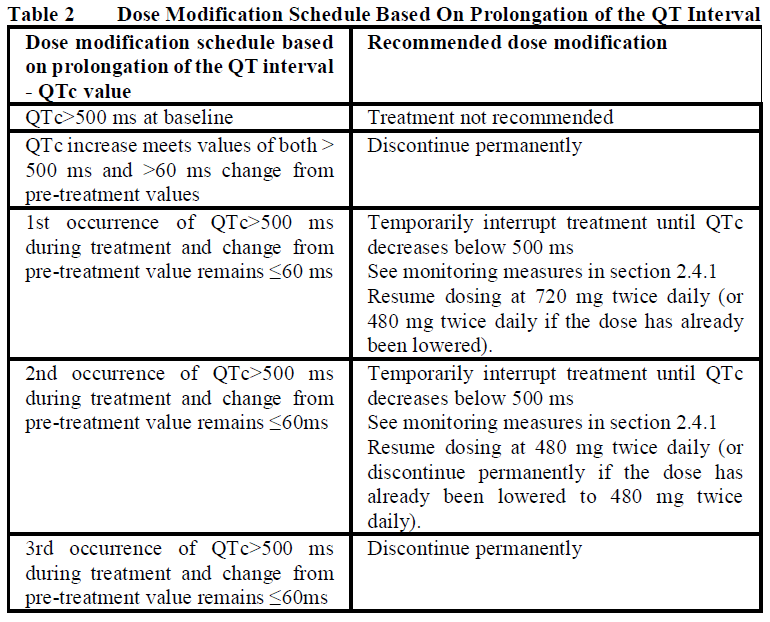 **2.2.1 Special Dosage Instructions** _Pediatric use:_ The safety and efficacy of Zelboraf in patients under the age of 18 have not been established. Zelboraf is not approved for use in patients under the age of 18 years (see section 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Geriatric use:_ No special dose adjustment of Zelboraf is required in patients aged > 65 years. _Renal impairment:_ There have been no specific renal impairment studies; based on retrospective analyses of data from clinical trials, no starting dose adjustment is required in patients with mild or moderate renal impairment. _Hepatic impairment:_ There have been no specific hepatic impairment studies; based on retrospective analyses of data from clinical trials, no starting dose adjustment is required in patients with mild or moderate hepatic impairment.
ORAL
Medical Information
**2.1 Therapeutic Indication(s)** Zelboraf® is indicated in monotherapy for the treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma (see section 3.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**2.3 Contraindications** Zelboraf is contraindicated in patients with known hypersensitivity to vemurafenib or to any of its excipients _(see section 2.4.1 General, Warnings and Precautions – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information)_.
L01XE15
xl 01 xe 15
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Delpharm Milano S.r.l.
Active Ingredients
Documents
Package Inserts
Zelboraf_PI.pdf
Approved: March 21, 2023
