Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**Recommended Dosage:** _**Non-small cell lung cancer (NSCLC)**_ _Monotherapy_ The recommended dose of gemcitabine is 1,000 mg/m2, given by 30-minute intravenous infusion. This should be repeated once weekly for 3 weeks, followed by a 1-week rest period. This 4-week cycle is then repeated. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. _Combination use_ Gemcitabine in combination with cisplatin has been investigated using two dosing regimen. One regimen used a 3-week schedule and the other used a 4-week schedule. The 3-week schedule used gemcitabine 1,250 mg/m2, given by 30-minute intravenous infusion, on days 1 and 8 of each 21-day cycle. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. The 4-week schedule used gemcitabine 1,000 mg/m2, given by 30-minute intravenous infusion, on days 1, 8, and 15 of each 28-day cycle. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. Cisplatin has been used at doses between 75–100 mg/m2 once every 3 or 4 weeks. _**Pancreatic cancer**_ _Monotherapy_ The recommended dose of gemcitabine is 1,000 mg/m2, given by 30-minute intravenous infusion. This should be repeated once weekly for up to 7 weeks, followed by a week of rest. Subsequent cycles should consist of injections once weekly for 3 consecutive weeks out of every 4 weeks. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. _**Bladder cancer**_ _Combination use_ The recommended dose for gemcitabine is 1,000 mg/m2, given by 30-minute infusion. The dose should be given on days 1, 8, and 15 of each 28-day cycle in combination with cisplatin. Cisplatin is given at a recommended dose of 70 mg/m2 on day 1 following gemcitabine or day 2 of each 28-day cycle. This 4-week cycle is then repeated. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. More myelosuppression when cisplatin was used in doses of 100 mg/m2. _**Breast cancer**_ _Combination use_ Gemcitabine in combination with paclitaxel is recommended using paclitaxel 175 mg/m2 administered on day 1 over approximately 3 hours as an intravenous infusion, followed by gemcitabine 1,250 mg/m2 as a 30-minute intravenous infusion on days 1 and 8 of each 21-day cycle. Dose reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. _**Monitoring for toxicity and dose modification due to toxicity**_ _Dose modification due to nonhematological toxicity_ Periodic physical examination and checks of renal and hepatic function should be made to detect nonhematological toxicity. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. In general, for severe (grade 3 or 4) nonhematological toxicity, except nausea/vomiting, therapy with gemcitabine should be withheld or decreased depending on the judgement of the treating physician. Doses should be withheld until toxicity has resolved in the opinion of the physician. For cisplatin and paclitaxel dosage adjustment in combination therapy, please refer to the corresponding manufacturers’ prescribing information. _Dose modification due to hematological toxicity_ Initiation of a cycle For all indications, the patient must be monitored before each dose for platelet and granulocyte counts. Patients should have an absolute granulocyte count of at least 1,500 (x 106/l) and platelet count of 100,000 (x 106/l) prior to the initiation of a cycle. Within a cycle Dose modifications of gemcitabine within a cycle should be performed according to the following tables: 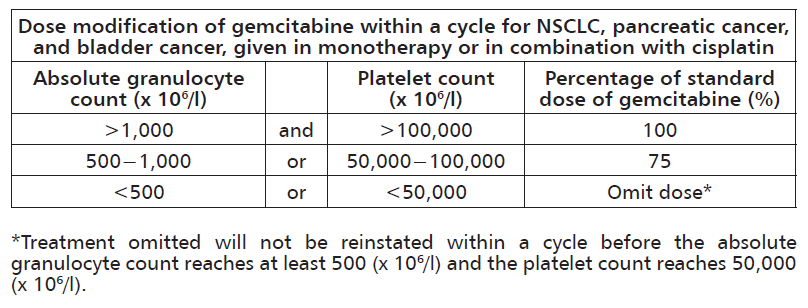 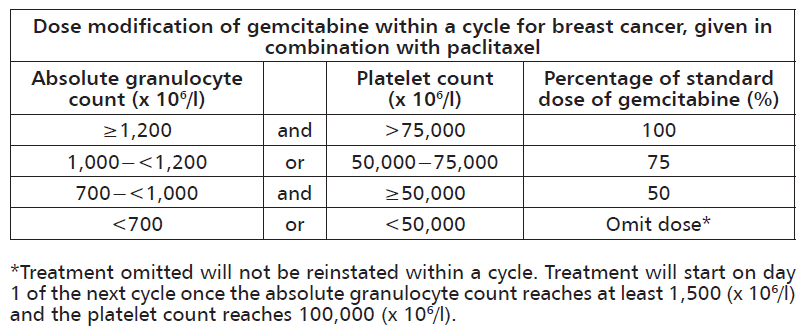 Dose modifications due to hematological toxicity in subsequent cycles, for all indications The gemcitabine dose should be reduced to 75% of the original cycle initiation dose, in the case of the following hematological toxicities: - Absolute granulocyte count <500 x 106/l for more than 5 days. - Absolute granulocyte count <100 x 106/l for more than 3 days. - Febrile neutropenia. - Platelets <25,000 x 106/l. - Cycle delay of more than 1 week due to toxicity. _**Special populations**_ _Patients with renal or hepatic impairment_ Gemcitabine should be used with caution in patients with hepatic or renal impairment as there is insufficient information to allow for clear dose recommendations for this patient populations (see **Warnings and Precautions** and **Pharmacokinetics** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose reduction is recommended in patients with elevated serum bilirubin concentration because such patients are at increased risk of toxicity. The toxicity was mostly related to the liver. Patients with elevated serum creatinine concentration appeared to experience increased sensitivity to gemcitabine. _Elderly population (>65 years)_ Gemcitabine has been well tolerated in patients over the age of 65. There is no evidence to suggest that dose adjustments, other than those already recommended for all patients, are necessary in elderly, although gemcitabine clearance and half-life are affected by age (see **Pharmacokinetics** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Pediatric population (<18 years)_ Gemcitabine is not recommended for use in children under 18 years of age due to insufficient data on safety and efficacy. _**Method of administration**_ Gemcitabine is tolerated well during infusion and may be administered ambulant. If extravasation occurs, generally the infusion must be stopped immediately and started again in another blood vessel. The patient should be monitored carefully after the administration.
INTRAVENOUS
Medical Information
**Indications:** _**Non-small cell lung cancer (NSCLC)**_ Gemcitabine, in combination with cisplatin, is indicated as a first line treatment of patients with locally advanced (inoperable stage IIIA or IIIB) or metastatic (stage IV) NSCLC. Gemcitabine is indicated for the palliative treatment of adult patients with locally advanced or metastatic NSCLC. _**Pancreatic cancer**_ Gemcitabine is indicated for the treatment of adult patients with locally advanced or metastatic adenocarcinoma of the pancreas. Gemcitabine is indicated for patients with 5-FU refractory pancreatic cancer. _**Bladder cancer**_ Gemcitabine is indicated for the treatment of advanced bladder cancer (muscle invasive stage IV tumors with or without metastases) in combination with cisplatin therapy. _**Breast cancer**_ Gemcitabine, in combination with paclitaxel is indicated for the treatment of patients with unresectable, locally recurrent or metastatic breast cancer who have relapsed following adjuvant/neoadjuvant chemotherapy. Prior chemotherapy should have included an anthracycline unless clinically contraindicated.
**Contraindications:** - Hypersensitivity to the active substance or to any of the excipients. - Breastfeeding (see **Use during Pregnancy and Lactation** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01BC05
gemcitabine
Manufacturer Information
GLORIOUS DEXA SINGAPORE PTE. LTD.
PT Fonko International Pharmaceuticals
Active Ingredients
Documents
Package Inserts
Fonko-Gemcitabine Lyo Powder_PI.pdf
Approved: May 3, 2023
