Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Adults The recommended dose for the treatment of community-acquired pneumonia due to the indicated susceptible microorganisms is of 500 mg administered as a single intravenous daily dose for at least two consecutive days. The intravenous therapy should be followed by the oral administration of azithromycin in a single daily dose of 500 mg up to 7 to 10 days of treatment. Transition to oral therapy should be carried out when indicated by the doctor and according to the clinical response. The recommended dose for the treatment of pelvic inflammatory disease (PID) due to the indicated susceptible microorganisms is of 500 mg administered as a single intravenous daily dose for one or two days. The intravenous therapy should be followed by the oral administration of azithromycin in a single daily dose of 250 mg up to 7 days of treatment. Transition to oral therapy should be carried out when indicated by the doctor and according to the clinical response. If anaerobic microorganisms are suspected of contributing to the infection, an antimicrobial anaerobic agent may be administered in combination with azithromycin. Children The efficacy and safety of IV azithromycin for the treatment of infections in children and adolescents has not been established. Use in the elderly The same dosage as recommended for adult patients is used in the elderly. Since elderly can be patients with ongoing proarrhythmic conditions a particular caution is recommended due to the risk of developing cardiac arrhythmia and torsades de pointes. (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Use in patients with renal impairment No dose adjustment is recommended in patients with mild to moderate renal impairment (GFR 10 – 80 ml/min). Caution should be exercised when azithromycin is administered to patients with severe renal impairment (GFR < 10 ml/min) (see section 4.4 and section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Use in patients with hepatic impairment Dose adjustment is not required for patients with mild to moderate hepatic dysfunction but the medicinal product should be used with caution in patients with significant hepatic diseases (see section 4.4 Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration Once the product is reconstituted and diluted, it is intended to be administered by intravenous infusion. It should not be administered as an intravenous bolus or an intramuscular injection (see section 4.4 and section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The concentration of the solution for infusion and the infusion rate of azithromycin as powder for solution for infusion should be 1 mg/ml over 3 hours or 2 mg/ml over 1 hour. Concentrations >2mg/ml should be avoided. Preparation of the solution for intravenous administration is as follows: _Reconstitution_ The initial solution of azithromycin is prepared by adding 4.8 ml of sterile water for injections to the 500 mg vial and shaking the vial until all the drug is dissolved. It is recommended that a standard 5 ml (non-automated) syringe be used to ensure that the exact volume of 4.8 ml of sterile water for injections is dispensed. Each ml of reconstituted solution contains azithromycin dihydrate equivalent to 100 mg azithromycin (100 mg/ml). Parenteral administration drugs should be inspected visually for particulate in suspension prior to administration. If particulate in suspension is evident in reconstituted solution, the drug solution should be discarded. The reconstituted solution must be further diluted prior to administration as instructed below. _Dilution_ To provide azithromycin over a concentration range of 1.0 – 2.0 mg/ml, transfer 5 ml of the 100 mg/ml azithromycin solution to the appropriate amount of any of the diluents listed in section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. 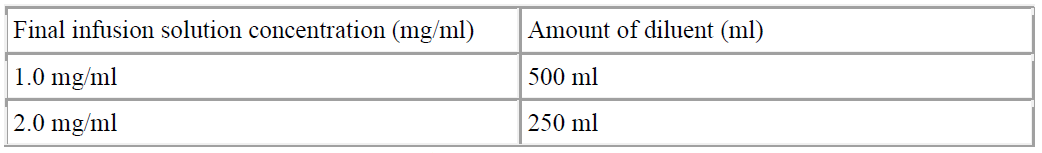 It is recommended that a 500 mg dose of azithromycin as powder for solution for infusion, diluted according to the instructions above, be administered as an intravenous infusion over at least 60 minutes.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** It is indicated for the treatment of community-acquired pneumonia due to _Chlamydia pneumoniae, Haemophilus influenzae, Legionella pneumophila, Moraxella catarrhalis, Mycoplasma pneumoniae_ or _Streptococcus pneumoniae_ in patients who require initial intravenous therapy. It is also indicated for the treatment of pelvic inflammatory disease due to _Chlamydia trachomatis_, _Neisseria gonorrhoeae_, or _Mycoplasma hominis_ in patients who require initial intravenous therapy.
**4.3 Contraindications** The use of the product is contraindicated in patients with a known hypersensitivity to azithromycin, erythromycin or any of the macrolide or ketolide antibiotics or to any excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Co-administration of macrolides with cisapride is contraindicated.
J01FA10
azithromycin
Manufacturer Information
GOLDPLUS UNIVERSAL PTE LTD
Anfarm Hellas S.A.
Active Ingredients
Documents
Package Inserts
SPC-Zithrotel inj clean.pdf
Approved: September 29, 2023
