Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**NEURORADIOLOGY**  **ANGIOGRAPHY** 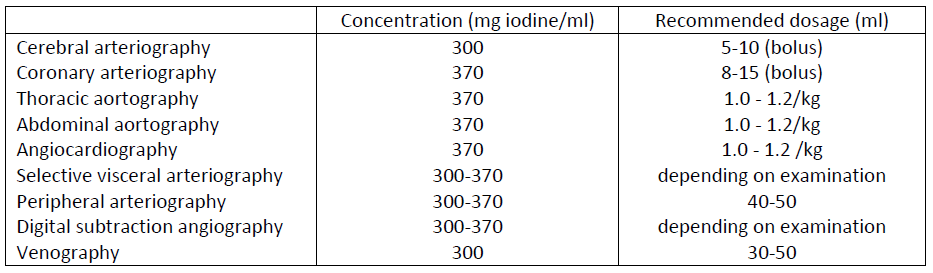 **UROGRAPHY** Iopamiro 300 and 370 should be used; the dose recommended for this type of examination is 30 to 50 ml. The less marked osmotic diuresis induced by the nonionic agent makes Iopamiro 370 especially suitable for patients with mild or moderately severe renal insufficiency and for neonates. The new contrast medium affords diagnostically useful nephrography even in patients with major renal insufficiency. **OTHER DIAGNOSTIC PROCEDURES** 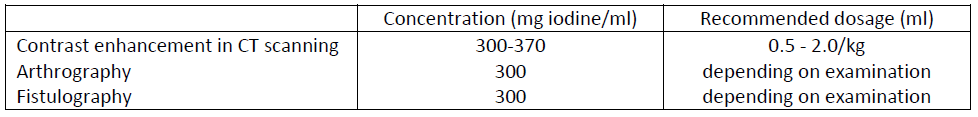 For the enhancement of contrast in CT scans Iopamiro may be injected intravenously as a bolus, as a drip infusion or by a combination of the two methods.
INTRAVENOUS, OTHERS
Medical Information
**INDICATIONS** _NEURORADIOLOGY:_ myeloradiculography, cisternography and ventriculography. _ANGIOGRAPHY:_ cerebral arteriography, coronary arteriography, thoracic aortography, abdominal aortography, angiocardiography, selective visceral arteriography, peripheral arteriography, venography, digital subtraction angiography (DSA), DSA of cerebral arteries, DSA of peripheral arteries, DSA of abdominal arteries. _UROGRAPHY:_ intravenous urography. _CONTRAST ENHANCEMENT IN CT SCANNING_. _ARTHROGRAPHY_. _FISTULOGRAPHY_.
**CONTRAINDICATIONS** There are no definite or absolute contraindications to the use of Iopamiro, with the possible exception of Waldenström's macroglobulinemia, multiple myeloma, and severe liver and kidney diseases.
V08AB04
iopamidol
Manufacturer Information
DCH AURIGA SINGAPORE
Patheon Italia S.p.A
Bracco SpA
Active Ingredients
Documents
Package Inserts
Iopamiro Injection PI.pdf
Approved: April 6, 2023
